This is a preprint.
Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2
- PMID: 32511299
- PMCID: PMC7217148
- DOI: 10.1101/2020.03.21.001628
Respiratory disease and virus shedding in rhesus macaques inoculated with SARS-CoV-2
Update in
-
Respiratory disease in rhesus macaques inoculated with SARS-CoV-2.Nature. 2020 Sep;585(7824):268-272. doi: 10.1038/s41586-020-2324-7. Epub 2020 May 12. Nature. 2020. PMID: 32396922 Free PMC article.
Abstract
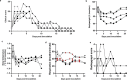
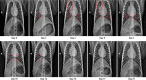
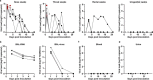
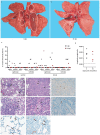
An outbreak of a novel coronavirus, now named SARS-CoV-2, causing respiratory disease and a ~2% case fatality rate started in Wuhan, China in December 2019. Following unprecedented rapid global spread, the World Health Organization declared COVID-19 a pandemic on March 11, 2020. Although data on disease in humans are emerging at a steady pace, certain aspects of the pathogenesis of SARS-CoV-2 can only be studied in detail in animal models, where repeated sampling and tissue collection is possible. Here, we show that SARS-CoV-2 causes respiratory disease in infected rhesus macaques, with disease lasting 8-16 days. Pulmonary infiltrates, a hallmark of human disease, were visible in lung radiographs of all animals. High viral loads were detected in swabs from the nose and throat of all animals as well as in bronchoalveolar lavages; in one animal we observed prolonged rectal shedding. Taken together, the rhesus macaque recapitulates moderate disease observed in the majority of human cases. The establishment of the rhesus macaque as a model of COVID-19 will increase our understanding of the pathogenesis of this disease and will aid development and testing of medical countermeasures.
Conflict of interest statement
Competing interests The authors declare no competing interests
Figures

References
-
- Organization, W. H. Coronavirus disease (COVID-2019) situation reports, <https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...>(2020).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
