This is a preprint.
Open Development and Clinical Validation Of Multiple 3D-Printed Sample-Collection Swabs: Rapid Resolution of a Critical COVID-19 Testing Bottleneck
- PMID: 32511491
- PMCID: PMC7273259
- DOI: 10.1101/2020.04.14.20065094
Open Development and Clinical Validation Of Multiple 3D-Printed Sample-Collection Swabs: Rapid Resolution of a Critical COVID-19 Testing Bottleneck
Update in
-
Open Development and Clinical Validation of Multiple 3D-Printed Nasopharyngeal Collection Swabs: Rapid Resolution of a Critical COVID-19 Testing Bottleneck.J Clin Microbiol. 2020 Jul 23;58(8):e00876-20. doi: 10.1128/JCM.00876-20. Print 2020 Jul 23. J Clin Microbiol. 2020. PMID: 32393482 Free PMC article.
Abstract
The SARS-CoV-2 pandemic has caused a severe international shortage of the nasopharyngeal swabs that are required for collection of optimal specimens, creating a critical bottleneck in the way of high-sensitivity virological testing for COVID-19. To address this crisis, we designed and executed an innovative, radically cooperative, rapid-response translational-research program that brought together healthcare workers, manufacturers, and scientists to emergently develop and clinically validate new swabs for immediate mass production by 3D printing. We performed a rigorous multi-step preclinical evaluation on 160 swab designs and 48 materials from 24 companies, laboratories, and individuals, and shared results and other feedback via a public data repository (http://github.com/rarnaout/Covidswab/). We validated four prototypes through an institutional review board (IRB)-approved clinical trial that involved 276 outpatient volunteers who presented to our hospital's drive-through testing center with symptoms suspicious for COVID-19. Each participant was swabbed with a reference swab (the control) and a prototype, and SARS-CoV-2 reverse-transcriptase polymerase chain reaction (RT-PCR) results were compared. All prototypes displayed excellent concordance with the control (κ=0.85-0.89). Cycle-threshold (Ct) values were not significantly different between each prototype and the control, supporting the new swabs' non-inferiority (Mann-Whitney U [MWU] p>0.05). Study staff preferred one of the prototypes over the others and the control swab overall. The total time elapsed between identification of the problem and validation of the first prototype was 22 days. Contact information for ordering can be found at http://printedswabs.org. Our experience holds lessons for the rapid development, validation, and deployment of new technology for this pandemic and beyond.
Figures
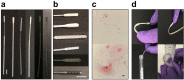
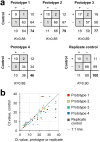
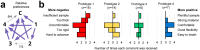
References
-
- COVID-19 Map - Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed April 21, 2020.
-
- Thomas K. Coronavirus Test Obstacles: A Shortage of Face Masks and Swabs. The New York Times; https://www.nytimes.com/2020/03/18/health/coronavirus-test-shortages-fac.... Published March 18, 2020. Accessed April 13, 2020.
-
- Kodzius R, Xiao K, Wu J, et al. Inhibitory effect of common microfluidic materials on PCR outcome. Sensors and Actuators B: Chemical. 2012;161(1):349–358. doi:10.1016/j.snb.2011.10.044 - DOI
-
- Bessetti J. An Introduction to PCR Inhibitors. 2007. https://www.promega.es/-/media/files/resources/profiles-in-dna/1001/an-i.... Accessed April 13, 2020.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
