Chronic defects in intraspinal mechanisms of spike encoding by spinal motoneurons following chemotherapy
- PMID: 32511953
- PMCID: PMC7937189
- DOI: 10.1016/j.expneurol.2020.113354
Chronic defects in intraspinal mechanisms of spike encoding by spinal motoneurons following chemotherapy
Abstract
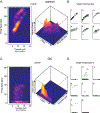
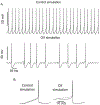
Chemotherapy-induced sensorimotor disabilities, including gait and balance disorders, as well as physical fatigue often persist for months and sometimes years into disease free survival from cancer. While associated with impaired sensory function, chronic sensorimotor disorders might also depend on chemotherapy-induced defects in other neuron types. In this report, we extend consideration to motoneurons, which, if chronically impaired, would necessarily degrade movement behavior. The present study was undertaken to determine whether motoneurons qualify as candidate contributors to chronic sensorimotor disability independently from sensory impairment. We tested this possibility in vivo from rats 5 weeks following human-scaled treatment with one of the platinum-based compounds, oxaliplatin, widely used in chemotherapy for a variety of cancers. Action potential firing of spinal motoneurons responding to different fixed levels of electrode-current injection was measured in order to assess the neurons' intrinsic capacity for stimulus encoding. The encoding of stimulus duration and intensity corroborated in untreated control rats was severely degraded in oxaliplatin treated rats, in which motoneurons invariably exhibited erratic firing that was unsustained, unpredictable from one stimulus trial to the next, and unresponsive to changes in current strength. Direct measurements of interspike oscillations in membrane voltage combined with computer modeling pointed to aberrations in subthreshold conductances as a plausible contributor to impaired firing behavior. These findings authenticate impaired spike encoding as a candidate contributor to, in the case of motoneurons, deficits in mobility and fatigue. Aberrant firing also becomes a deficit worthy of testing in other CNS neurons as a potential contributor to perceptual and cognitive disorders induced by chemotherapy in patients.
Keywords: CNS; Central nervous system; Chemotherapy; Computer modeling; Encoding; Firing behavior; Impaired excitability; Motoneurons; Rate-modulation; Subthreshold oscillations.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures


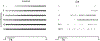


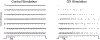

References
-
- Bennett BK et al. Impact of oxaliplatin-induced neuropathy: a patient perspective. Supportive Care in Cancer 20, 2959–2967 (2012). - PubMed
-
- Marshall TF, Zipp GP, Battaglia F, Moss R. & Bryan S. Chemotherapy-induced-peripheral neuropathy, gait and fall risk in older adults following cancer treatment. Journal of Cancer Research and Practice (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

