Layer-dependent functional connectivity methods
- PMID: 32512115
- PMCID: PMC11800141
- DOI: 10.1016/j.pneurobio.2020.101835
Layer-dependent functional connectivity methods
Abstract
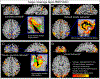
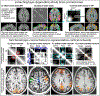
Recent methodological advances in fMRI contrast and readout strategies have allowed researchers to approach the mesoscopic spatial regime of cortical layers. This has revolutionized the ability to map cortical information processing within and across brain systems. However, until recently, most layer-fMRI studies have been confined to primary cortices using basic block-design tasks and macro-vascular-contaminated sequence contrasts. To become an established method for user-friendly applicability in neuroscience practice, layer-fMRI acquisition and analysis methods need to be extended to more flexible connectivity-based experiment designs; they must be able to capture subtle changes in brain networks of higher-order cognitive areas, and they should not be spatially biased with unwanted vein signals. In this article, we review the most pressing challenges of layer-dependent fMRI for large-scale neuroscientific applicability and describe recently developed acquisition methodologies that can resolve them. In doing so, we review technical tradeoffs and capabilities of modern MR-sequence approaches to achieve measurements that are free of locally unspecific vein signal, with whole-brain coverage, sub-second sampling, high resolutions, and with a combination of those capabilities. The presented approaches provide whole-brain layer-dependent connectivity data that open a new window to investigate brain network connections. We exemplify this by reviewing a number of candidate tools for connectivity analyses that will allow future studies to address new questions in network neuroscience. The considered network analysis tools include: hierarchy mapping, directional connectomics, source-specific connectivity mapping, and network sub-compartmentalization. We conclude: Whole-brain layer-fMRI without large-vessel contamination is applicable for human neuroscience and opens the door to investigate biological mechanisms behind any number of psychological and psychiatric phenomena, such as selective attention, hallucinations and delusions, and even conscious perception.
Keywords: 7T fMRI; CBV-fMRI; Functional connectivity; Human connectome; Laminar fMRI; Mesoscopic fMRI; VASO; Whole brain submillimeter fMRI; layer-fMRI.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures









References
-
- Beckmann CF and Smith SM (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23:137–152. - PubMed
-
- Bullmore E and Sporns O (2009). Complex brain networks: Graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience, 10(3):186–198. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

