Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study
- PMID: 32512811
- PMCID: PMC7357031
- DOI: 10.3390/jcm9061738
Circulating Tumor DNA as a Preoperative Marker of Recurrence in Patients with Peritoneal Metastases of Colorectal Cancer: A Clinical Feasibility Study
Abstract
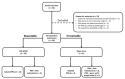
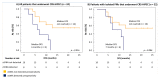
Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) may be curative for colorectal cancer patients with peritoneal metastases (PMs) but it has a high rate of morbidity. Accurate preoperative patient selection is therefore imperative, but is constrained by the limitations of current imaging techniques. In this pilot study, we explored the feasibility of circulating tumor (ct) DNA analysis to select patients for CRS-HIPEC. Thirty patients eligible for CRS-HIPEC provided blood samples preoperatively and during follow-up if the procedure was completed. Targeted Next-Generation Sequencing (NGS) of DNA from PMs was used to identify bespoke mutations that were subsequently tested in corresponding plasma cell-free (cf) DNA samples using droplet digital (dd) PCR. CtDNA was detected preoperatively in cfDNA samples from 33% of patients and was associated with a reduced disease-free survival (DFS) after CRS-HIPEC (median 6.0 months vs median not reached, p = 0.016). This association could indicate the presence of undiagnosed systemic metastases or an increased metastatic potential of the tumors. We demonstrate the feasibility of ctDNA to serve as a preoperative marker of recurrence in patients with PMs of colorectal cancer using a highly sensitive technique. A more appropriate treatment for patients with preoperative ctDNA detection may be systemic chemotherapy in addition to, or instead of, CRS-HIPEC.
Keywords: CRS-HIPEC; circulating tumor DNA; colorectal cancer; droplet digital PCR; liquid biopsy; peritoneal metastases.
Conflict of interest statement
DAMH has been on the speakers’ bureau of QIAGEN, serves occasionally on the scientific advisory boards of Pfizer and Bristol-Myers Squibb, and is minority shareholder of Self-screen B.V., a spin-off company of Amsterdam UMC, Vrije Universiteit Amsterdam. No other authors declare a conflict of interest.
Figures


Similar articles
-
Treatment of Isolated Peritoneal Recurrences in Patients with Colorectal Peritoneal Metastases Previously Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy.Ann Surg Oncol. 2018 Jul;25(7):1992-2001. doi: 10.1245/s10434-018-6423-8. Epub 2018 Apr 18. Ann Surg Oncol. 2018. PMID: 29671139
-
Treatment of ovarian metastases of colorectal and appendiceal carcinoma in the era of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.Eur J Surg Oncol. 2014 Aug;40(8):937-42. doi: 10.1016/j.ejso.2014.02.238. Epub 2014 Feb 28. Eur J Surg Oncol. 2014. PMID: 24630923
-
Cytoreductive Surgery With or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer With Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis.J Clin Oncol. 2019 Aug 10;37(23):2028-2040. doi: 10.1200/JCO.18.01688. Epub 2019 May 14. J Clin Oncol. 2019. PMID: 31084544
-
[Surgical treatment of peritoneal metastases of colorectal cancer].Chirurg. 2018 Sep;89(9):663-668. doi: 10.1007/s00104-018-0620-7. Chirurg. 2018. PMID: 29589077 Review. German.
-
Patient selection for cytoreductive surgery and HIPEC for the treatment of peritoneal metastases from colorectal cancer.Cancer Manag Res. 2017 Jun 30;9:259-266. doi: 10.2147/CMAR.S119569. eCollection 2017. Cancer Manag Res. 2017. PMID: 28721098 Free PMC article. Review.
Cited by
-
Effective Strategies to Predict Survival of Colorectal Peritoneal Metastases Patients Eligible for Cytoreductive Surgery and HIPEC.Cancer Manag Res. 2021 Jul 1;13:5239-5249. doi: 10.2147/CMAR.S277912. eCollection 2021. Cancer Manag Res. 2021. PMID: 34234566 Free PMC article. Review.
-
Practical recommendations for using ctDNA in clinical decision making.Nature. 2023 Jul;619(7969):259-268. doi: 10.1038/s41586-023-06225-y. Epub 2023 Jul 12. Nature. 2023. PMID: 37438589
-
Circulating Tumor DNA and Management of Colorectal Cancer.Cancers (Basel). 2023 Dec 19;16(1):21. doi: 10.3390/cancers16010021. Cancers (Basel). 2023. PMID: 38201448 Free PMC article. Review.
-
Clinical Applications of Circulating Tumor DNA Profiling in GI Cancers.JCO Oncol Pract. 2024 Nov;20(11):1481-1490. doi: 10.1200/OP.24.00167. Epub 2024 Nov 12. JCO Oncol Pract. 2024. PMID: 39531845 Review.
-
The Landscape of ctDNA in Appendiceal Adenocarcinoma.Clin Cancer Res. 2025 Feb 3;31(3):551-560. doi: 10.1158/1078-0432.CCR-24-2474. Clin Cancer Res. 2025. PMID: 39679931 Free PMC article.
References
-
- Franko J., Shi Q., Meyers J.P., Maughan T.S., Adams R.A., Seymour M.T., Saltz L., Punt C.J., Koopman M., Tournigand C., et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17:1709–1719. doi: 10.1016/S1470-2045(16)30500-9. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

