Regulation of Deubiquitinating Enzymes by Post-Translational Modifications
- PMID: 32512887
- PMCID: PMC7312083
- DOI: 10.3390/ijms21114028
Regulation of Deubiquitinating Enzymes by Post-Translational Modifications
Abstract
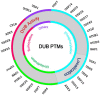
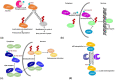
Ubiquitination and deubiquitination play a critical role in all aspects of cellular processes, and the enzymes involved are tightly regulated by multiple factors including posttranslational modifications like most other proteins. Dysfunction or misregulation of these enzymes could have dramatic physiological consequences, sometimes leading to diseases. Therefore, it is important to have a clear understanding of these regulatory processes. Here, we have reviewed the posttranslational modifications of deubiquitinating enzymes and their consequences on the catalytic activity, stability, abundance, localization, and interaction with the partner proteins.
Keywords: activity; deubiquitinase (DUB); deubiquitinating enzyme; disease; interaction; localization; post-translational modification (PTM).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

