Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction
- PMID: 32513195
- PMCID: PMC7281956
- DOI: 10.1186/s12989-020-00353-3
Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction
Abstract
Background: Wide applications of nanoparticles (NPs) have raised increasing concerns about safety to humans. Oxidative stress and inflammation are extensively investigated as mechanisms for NPs-induced toxicity. Autophagy and lysosomal dysfunction are emerging molecular mechanisms. Inhalation is one of the main pathways of exposing humans to NPs, which has been reported to induce severe pulmonary inflammation. However, the underlying mechanisms and, more specifically, the interplays of above-mentioned mechanisms in NPs-induced pulmonary inflammation are still largely obscure. Considered that NPs exposure in modern society is often unavoidable, it is highly desirable to develop effective strategies that could help to prevent nanomaterials-induced pulmonary inflammation.
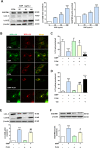
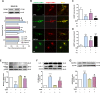
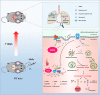
Results: Pulmonary inflammation induced by intratracheal instillation of silica nanoparticles (SiNPs) in C57BL/6 mice was prevented by PJ34, a poly (ADP-ribose) polymerase (PARP) inhibitor. In human lung bronchial epithelial (BEAS-2B) cells, exposure to SiNPs reduced cell viability, and induced ROS generation, impairment in lysosome function and autophagic flux. Inhibition of ROS generation, PARP and TRPM2 channel suppressed SiNPs-induced lysosome impairment and autophagy dysfunction and consequent inflammatory responses. Consistently, SiNPs-induced pulmonary inflammation was prevented in TRPM2 deficient mice.
Conclusion: The ROS/PARP/TRPM2 signaling is critical in SiNPs-induced pulmonary inflammation, providing novel mechanistic insights into NPs-induced lung injury. Our study identifies TRPM2 channel as a new target for the development of preventive and therapeutic strategies to mitigate nanomaterials-induced lung inflammation.
Keywords: Autophagy dysfunction; Lysosomal impairment; Nanoparticles; Pulmonary inflammation; ROS/PARP/TRPM2 signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Yang H, Wu QY, Li MY, Lao CS, Zhang YJ. Pulmonary toxicity in rats caused by exposure to Intratracheal instillation of SiO2 nanoparticles. Biomed Environ Sci. 2017;30(4):264–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

