From COVID-19 research to vaccine application: why might it take 17 months not 17 years and what are the wider lessons?
- PMID: 32513202
- PMCID: PMC7276964
- DOI: 10.1186/s12961-020-00571-3
From COVID-19 research to vaccine application: why might it take 17 months not 17 years and what are the wider lessons?
Abstract
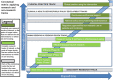
It is often said that it takes 17 years to move medical research from bench to bedside. In a coronavirus disease (COVID-19) world, such time-lags feel intolerable. In these extraordinary circumstances could years be made into months? If so, could those lessons be used to accelerate medical research when the crisis eases?To measure time-lags in health and biomedical research as well as to identify ways of reducing them, we developed and published (in 2015) a matrix consisting of overlapping tracks (or stages/phases) in the translation from discovery research to developed products, policies and practice. The matrix aids analysis by highlighting the time and actions required to develop research (and its translation) both (1) along each track and (2) from one track to another, e.g. from the discovery track to the research-in-humans track. We noted four main approaches to reducing time-lags, namely increasing resources, working in parallel, starting or working at risk, and improving processes.Examining these approaches alongside the matrix helps interpret the enormous global effort to develop a vaccine for the 2019 novel coronavirus SARS-CoV-2, the causative agent of COVID-19. Rapid progress in the discovery/basic and human research tracks is being made through a combination of large-scale funding, work being conducted in parallel (between different teams globally and through working in overlapping tracks), working at greater (but proportionate) risk to safety than usual, and adopting various new processes. The overlapping work of some of the teams involves continuing animal research whilst entering vaccine candidates into Phase I trials alongside planning their Phase II trials. The additional funding available helps to reduce some of the usual financial risks in moving so quickly. Going forward through the increasingly large human trials for safety, dosage and efficacy, it will be vital to overlap work in parallel in the often challenging public policy and clinical tracks. Thus, regulatory and reimbursement bodies are beginning and preparing rapid action to pull vaccines proving to be safe and effective through to extraordinarily rapid application to the general population. Monitoring the development of a COVID-19 vaccine using the matrix (modified as necessary) could help identify which of the approaches speeding development and deployment could be usefully applied more widely in the future.
Keywords: COVID-19; Coronavirus disease; Matrix; Pandemic; Research translation; SARS-CoV-2; Time-lags; Timescales; Trials; Vaccine; World Health Organization.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Buxton M, Hanney S, Morris S, Sundmacher L, Mestre-Ferrandiz J, Garau M, Sussex J, Grant J, Ismail S, Nason E, Wooding S. Medical research: what’s it worth? London: Medical Research Council, Wellcome Trust and Academy of Medical Sciences; 2008.
-
- Balas E, Boren S. Managing clinical knowledge for health care improvement. In: van Bemmel JH, Mccray AT, editors. Yearbook of medical informatics. Stuttgart: Schattauer Verlagsgesellschaft mbH; 2000. pp. 65–70. - PubMed
-
- Grant J, Green L, Mason B. Basic research and health: a reassessment of the scientific basis for the support of biomedical science. Res Eval. 2003;12:217–224. doi: 10.3152/147154403781776618. - DOI
-
- Wratschko K. Empirical setting: the pharmaceutical industry. strategic orientation and alliance portfolio configuration. New York: Springer; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous