Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease
- PMID: 32513301
- PMCID: PMC7282036
- DOI: 10.1186/s40168-020-00868-z
Bacteroides thetaiotaomicron-derived outer membrane vesicles promote regulatory dendritic cell responses in health but not in inflammatory bowel disease
Abstract
Background: Bacteroides thetaiotaomicron (Bt) is a prominent member of the human intestinal microbiota that, like all gram-negative bacteria, naturally generates nanosized outer membrane vesicles (OMVs) which bud off from the cell surface. Importantly, OMVs can cross the intestinal epithelial barrier to mediate microbe-host cell crosstalk involving both epithelial and immune cells to help maintain intestinal homeostasis. Here, we have examined the interaction between Bt OMVs and blood or colonic mucosa-derived dendritic cells (DC) from healthy individuals and patients with Crohn's disease (CD) or ulcerative colitis (UC).
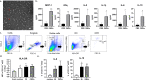
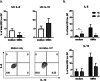
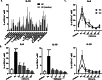
Results: In healthy individuals, Bt OMVs stimulated significant (p < 0.05) IL-10 expression by colonic DC, whereas in peripheral blood-derived DC they also stimulated significant (p < 0.001 and p < 0.01, respectively) expression of IL-6 and the activation marker CD80. Conversely, in UC Bt OMVs were unable to elicit IL-10 expression by colonic DC. There were also reduced numbers of CD103+ DC in the colon of both UC and CD patients compared to controls, supporting a loss of regulatory DC in both diseases. Furthermore, in CD and UC, Bt OMVs elicited a significantly lower proportion of DC which expressed IL-10 (p < 0.01 and p < 0.001, respectively) in blood compared to controls. These alterations in DC responses to Bt OMVs were seen in patients with inactive disease, and thus are indicative of intrinsic defects in immune responses to this commensal in inflammatory bowel disease (IBD).
Conclusions: Overall, our findings suggest a key role for OMVs generated by the commensal gut bacterium Bt in directing a balanced immune response to constituents of the microbiota locally and systemically during health which is altered in IBD patients. Video Abstract.
Keywords: Bacteroides thetaiotaomicron; Dendritic cells; Inflammatory bowel disease; Interleukin-10; Outer membrane vesicles.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Luckey TD. Introduction to intestinal microecology. Am. J. Clin. Nutr. 1972;25:1292–1294. - PubMed
Publication types
MeSH terms
Grants and funding
- BB/J004529/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10356/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10353/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10355/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

