Kalirin and Trio: RhoGEFs in Synaptic Transmission, Plasticity, and Complex Brain Disorders
- PMID: 32513570
- PMCID: PMC7321888
- DOI: 10.1016/j.tins.2020.05.002
Kalirin and Trio: RhoGEFs in Synaptic Transmission, Plasticity, and Complex Brain Disorders
Abstract
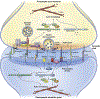
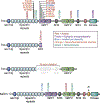
Changes in the actin cytoskeleton are a primary mechanism mediating the morphological and functional plasticity that underlies learning and memory. The synaptic Ras homologous (Rho) guanine nucleotide exchange factors (GEFs) Kalirin and Trio have emerged as central regulators of actin dynamics at the synapse. The increased attention surrounding Kalirin and Trio stems from the growing evidence for their roles in the etiology of a wide range of neurodevelopmental and neurodegenerative disorders. In this Review, we discuss recent findings revealing the unique and diverse functions of these paralog proteins in neurodevelopment, excitatory synaptic transmission, and plasticity. We additionally survey the growing literature implicating these proteins in various neurological disorders.
Keywords: Kalirin; RhoGEF; actin; autism; neurodegeneration; neurotransmission; schizophrenia; synaptic plasticity; trio.
Published by Elsevier Ltd.
Conflict of interest statement
Disclaimer Statement
The authors declare no competing interests.
Figures

References
-
- Schmidt A and Hall A, Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev, 2002. 16(13): p. 1587–609. - PubMed
-
- Wennerberg K, Rossman KL, and Der CJ, The Ras superfamily at a glance. J Cell Sci, 2005. 118(Pt 5): p. 843–6. - PubMed
-
- Etienne-Manneville S and Hall A, Rho GTPases in cell biology. Nature, 2002. 420(6916): p. 629–35. - PubMed
-
- Nowak JM, et al., [The Rho protein family and its role in the cellular cytoskeleton]. Postepy Hig Med Dosw (Online), 2008. 62: p. 110–7. - PubMed
-
- Ridley AJ, Rho family proteins: coordinating cell responses. Trends Cell Biol, 2001. 11(12): p. 471–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

