Acute Inflammation Alters Brain Energy Metabolism in Mice and Humans: Role in Suppressed Spontaneous Activity, Impaired Cognition, and Delirium
- PMID: 32513828
- PMCID: PMC7363463
- DOI: 10.1523/JNEUROSCI.2876-19.2020
Acute Inflammation Alters Brain Energy Metabolism in Mice and Humans: Role in Suppressed Spontaneous Activity, Impaired Cognition, and Delirium
Abstract
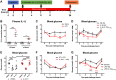
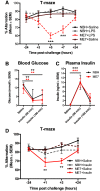
Systemic infection triggers a spectrum of metabolic and behavioral changes, collectively termed sickness behavior, which while adaptive, can affect mood and cognition. In vulnerable individuals, acute illness can also produce profound, maladaptive, cognitive dysfunction including delirium, but our understanding of delirium pathophysiology remains limited. Here, we used bacterial lipopolysaccharide (LPS) in female C57BL/6J mice and acute hip fracture in humans to address whether disrupted energy metabolism contributes to inflammation-induced behavioral and cognitive changes. LPS (250 µg/kg) induced hypoglycemia, which was mimicked by interleukin (IL)-1β (25 µg/kg) but not prevented in IL-1RI-/- mice, nor by IL-1 receptor antagonist (IL-1RA; 10 mg/kg). LPS suppression of locomotor activity correlated with blood glucose concentrations, was mitigated by exogenous glucose (2 g/kg), and was exacerbated by 2-deoxyglucose (2-DG) glycolytic inhibition, despite preventing IL-1β synthesis. Using the ME7 model of chronic neurodegeneration in female mice, to examine vulnerability of the diseased brain to acute stressors, we showed that LPS (100 µg/kg) produced acute cognitive dysfunction, selectively in those animals. These acute cognitive impairments were mimicked by insulin (11.5 IU/kg) and mitigated by glucose, demonstrating that acutely reduced glucose metabolism impairs cognition selectively in the vulnerable brain. To test whether these acute changes might predict altered carbohydrate metabolism during delirium, we assessed glycolytic metabolite levels in CSF in humans during inflammatory trauma-induced delirium. Hip fracture patients showed elevated CSF lactate and pyruvate during delirium, consistent with acutely altered brain energy metabolism. Collectively, the data suggest that disruption of energy metabolism drives behavioral and cognitive consequences of acute systemic inflammation.SIGNIFICANCE STATEMENT Acute systemic inflammation alters behavior and produces disproportionate effects, such as delirium, in vulnerable individuals. Delirium has serious short and long-term sequelae but mechanisms remain unclear. Here, we show that both LPS and interleukin (IL)-1β trigger hypoglycemia, reduce CSF glucose, and suppress spontaneous activity. Exogenous glucose mitigates these outcomes. Equivalent hypoglycemia, induced by lipopolysaccharide (LPS) or insulin, was sufficient to trigger cognitive impairment selectively in animals with existing neurodegeneration and glucose also mitigated those impairments. Patient CSF from inflammatory trauma-induced delirium also shows altered brain carbohydrate metabolism. The data suggest that the degenerating brain is exquisitely sensitive to acute behavioral and cognitive consequences of disrupted energy metabolism. Thus "bioenergetic stress" drives systemic inflammation-induced dysfunction. Elucidating this may offer routes to mitigating delirium.
Keywords: IL-1; cognitive; delirium; dementia; hypoglycemia; sepsis.
Copyright © 2020 Kealy, Murray et al.
Figures




Comment in
-
This Is Your Brain on (Low) Glucose.Trends Neurosci. 2020 Dec;43(12):933-935. doi: 10.1016/j.tins.2020.09.002. Epub 2020 Sep 17. Trends Neurosci. 2020. PMID: 32951858 Free PMC article.
References
-
- American Psychiatric Association (2013) Neurocognitive disorders - delirium. In: Diagnostic and statistical manual of mental disorders, Ed 5, pp 596–601. Washington, DC: American Psychiatric Association.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
