Clinical Performance of SARS-CoV-2 Molecular Tests
- PMID: 32513858
- PMCID: PMC7383556
- DOI: 10.1128/JCM.00995-20
Clinical Performance of SARS-CoV-2 Molecular Tests
Abstract
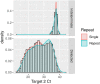
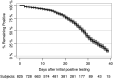
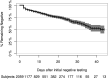
Molecular testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the gold standard for diagnosis of coronavirus disease 2019 (COVID-19), but the clinical performance of these tests is still poorly understood, particularly with regard to disease course, patient-specific factors, and viral shedding. From 10 March to 1 May 2020, NewYork-Presbyterian laboratories performed 27,377 SARS-CoV-2 molecular assays from 22,338 patients. Repeat testing was performed for 3,432 patients, of which 2,413 had initial negative and 802 had initial positive results. Repeat-tested patients were more likely to have severe disease and low viral loads. The negative predictive value of the first-day result among repeat-tested patients was 81.3% The clinical sensitivity of SARS-CoV-2 molecular assays was estimated between 58% and 96%, depending on the unknown number of false-negative results in single-tested patients. Conversion to negative was unlikely to occur before 15 to 20 days after initial testing or 20 to 30 days after the onset of symptoms, with 50% conversion occurring at 28 days after initial testing. Conversion from first-day negative to positive results increased linearly with each day of testing, reaching 25% probability in 20 days. Sixty patients fluctuated between positive and negative results over several weeks, suggesting that caution is needed when single-test results are acted upon. In summary, our study provides estimates of the clinical performance of SARS-CoV-2 molecular assays and suggests time frames for appropriate repeat testing, namely, 15 to 20 days after a positive test and the same day or next 2 days after a negative test for patients with high suspicion for COVID-19.
Keywords: COVID-19; SARS-CoV-2; laboratory utilization; negative predictive value; sensitivity.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Wu F, Zhao S, Yu B, Chen Y-M, Wang W, Song Z-G, Hu Y, Tao Z-W, Tian J-H, Pei Y-Y, Yuan M-L, Zhang Y-L, Dai F-H, Liu Y, Wang Q-M, Zheng J-J, Xu L, Holmes EC, Zhang Y-Z. 2020. A new coronavirus associated with human respiratory disease in China. Nature 579:265–269. doi:10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Zhu N, China Novel Coronavirus Investigating and Research Team, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733. doi:10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. 2020. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. doi:10.1016/S0140-6736(20)30566-3. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

