Anodal block permits directional vagus nerve stimulation
- PMID: 32513973
- PMCID: PMC7280203
- DOI: 10.1038/s41598-020-66332-y
Anodal block permits directional vagus nerve stimulation
Abstract
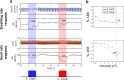
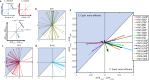
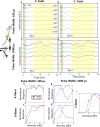
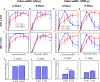
Vagus nerve stimulation (VNS) is a bioelectronic therapy for disorders of the brain and peripheral organs, and a tool to study the physiology of autonomic circuits. Selective activation of afferent or efferent vagal fibers can maximize efficacy and minimize off-target effects of VNS. Anodal block (ABL) has been used to achieve directional fiber activation in nerve stimulation. However, evidence for directional VNS with ABL has been scarce and inconsistent, and it is unknown whether ABL permits directional fiber activation with respect to functional effects of VNS. Through a series of vagotomies, we established physiological markers for afferent and efferent fiber activation by VNS: stimulus-elicited change in breathing rate (ΔBR) and heart rate (ΔHR), respectively. Bipolar VNS trains of both polarities elicited mixed ΔHR and ΔBR responses. Cathode cephalad polarity caused an afferent pattern of responses (relatively stronger ΔBR) whereas cathode caudad caused an efferent pattern (stronger ΔHR). Additionally, left VNS elicited a greater afferent and right VNS a greater efferent response. By analyzing stimulus-evoked compound nerve potentials, we confirmed that such polarity differences in functional responses to VNS can be explained by ABL of A- and B-fiber activation. We conclude that ABL is a mechanism that can be leveraged for directional VNS.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

