Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19)
- PMID: 32514047
- PMCID: PMC7278239
- DOI: 10.1038/s41418-020-0572-6
Expansion of myeloid-derived suppressor cells in patients with severe coronavirus disease (COVID-19)
Abstract
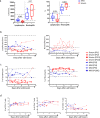
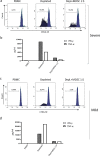
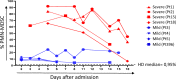
SARS-CoV-2 is associated with a 3.4% mortality rate in patients with severe disease. The pathogenesis of severe cases remains unknown. We performed an in-depth prospective analysis of immune and inflammation markers in two patients with severe COVID-19 disease from presentation to convalescence. Peripheral blood from 18 SARS-CoV-2-infected patients, 9 with severe and 9 with mild COVID-19 disease, was obtained at admission and analyzed for T-cell activation profile, myeloid-derived suppressor cells (MDSCs) and cytokine profiles. MDSC functionality was tested in vitro. In four severe and in four mild patients, a longitudinal analysis was performed daily from the day of admission to the early convalescent phase. Early after admission severe patients showed neutrophilia, lymphopenia, increase in effector T cells, a persisting higher expression of CD95 on T cells, higher serum concentration of IL-6 and TGF-β, and a cytotoxic profile of NK and T cells compared with mild patients, suggesting a highly engaged immune response. Massive expansion of MDSCs was observed, up to 90% of total circulating mononuclear cells in patients with severe disease, and up to 25% in the patients with mild disease; the frequency decreasing with recovery. MDSCs suppressed T-cell functions, dampening excessive immune response. MDSCs decline at convalescent phase was associated to a reduction in TGF-β and to an increase of inflammatory cytokines in plasma samples. Substantial expansion of suppressor cells is seen in patients with severe COVID-19. Further studies are required to define their roles in reducing the excessive activation/inflammation, protection, influencing disease progression, potential to serve as biomarkers of disease severity, and new targets for immune and host-directed therapeutic approaches.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- WHO. Novel coronavirus—China. http://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en (2020). Accessed 19 Jan 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

