Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer
- PMID: 32514151
- PMCID: PMC7314664
- DOI: 10.1038/s41388-020-1341-1
Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer
Abstract
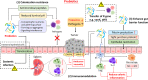
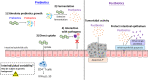
Research about the role of gut microbiome in colorectal cancer (CRC) is a newly emerging field of study. Gut microbiota modulation, with the aim to reverse established microbial dysbiosis, is a novel strategy for prevention and treatment of CRC. Different strategies including probiotics, prebiotics, postbiotics, antibiotics, and fecal microbiota transplantation (FMT) have been employed. Although these strategies show promising results, mechanistically by correcting microbiota composition, modulating innate immune system, enhancing gut barrier function, preventing pathogen colonization and exerting selective cytotoxicity against tumor cells, it should be noted that they are accompanied by risks and controversies that can potentially introduce clinical complications. During bench-to-bedside translation, evaluation of risk-and-benefit ratio, as well as patient selection, should be carefully performed. In view of the individualized host response to gut microbiome intervention, developing personalized microbiome therapy may be the key to successful clinical treatment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. - PubMed
-
- Kroemer G, Zitvogel L. Cancer immunotherapy in 2017: the breakthrough of the microbiota. Nat Rev Immunol. 2018;18:87–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

