Airway microbial diversity is decreased in young children with cystic fibrosis compared to healthy controls but improved with CFTR modulation
- PMID: 32514485
- PMCID: PMC7267737
- DOI: 10.1016/j.heliyon.2020.e04104
Airway microbial diversity is decreased in young children with cystic fibrosis compared to healthy controls but improved with CFTR modulation
Abstract
Background: Culture-independent next generation sequencing has identified diverse microbial communities within the cystic fibrosis (CF) airway. The study objective was to test for differences in the upper airway microbiome of children with CF and healthy controls and age-related differences in children with CF.
Methods: Oropharyngeal swabs and clinical data were obtained from 25 children with CF and 50 healthy controls aged ≤6 years. Bacterial DNA was amplified and sequenced for the V4 region of 16S rRNA marker-gene. Alpha diversity was measured using operational taxonomic units (OTUs), Shannon diversity, and the inverse Simpson's index. Beta diversity was measured using Morisita-Horn and Bray-Curtis and Jaccard distances. General linear models were used for comparison of alpha diversity measures between groups to account for differences in demographics and exposures. Mixed effects general linear models were used for longitudinal comparisons 1) between children with CF of different ages and 2) between children with CF receiving CF transmembrane conductance regulator (CFTR) modulators, children with CF not receiving CFTR modulators, and healthy controls to adjust for repeated measures per subject.
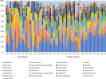
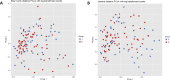
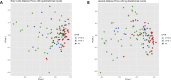
Results: Children with CF were more likely to have received antibiotics in the prior year than healthy controls (92% vs 24%, p < 0.001). Controlling age, race, ethnicity, length of breastfeeding, and having siblings, children with CF had a lower richness than healthy controls: OTUs 62.1 vs 83, p = 0.022; and trended toward lower diversity: Shannon 2.09 vs 2.35, p = 0.057; inverse Simpson 5.7 vs 6.92, p = 0.118. Staphylococcus, three Rothia OTUs, and two Streptococcus OTUs were more abundant in CF children versus healthy controls (all p < 0.05). Bray-Curtis and Jaccard distances, which reflect overall microbial community composition, were also significantly different (both p = 0.001). In longitudinally collected samples from children with CF, Morisita-Horn trended toward more similarity in those aged 0-2 years compared to those aged 3-6 years (p = 0.070). In children >2 years of age, there was a significant trend in increasing alpha diversity measures between children with CF not receiving CFTR modulators, children with CF receiving CFTR modulators, and healthy controls: OTUs 63.7 vs 74.7 vs 97.6, p < 0.001; Shannon 2.11 vs 2.34 vs 2.56, p < 0.001; inverse Simpson 5.78 vs 7.23 vs 7.96, p < 0.001.
Conclusions: Children with CF have lower bacterial diversity and different composition of organisms compared with healthy controls. This appears to start in early childhood, is possibly related to the use of antibiotics, and may be partially corrected with the use of CFTR modulators.
Keywords: Cystic fibrosis; Epidemiology; Health sciences; Human microbiome; Infectious disease; Microbiology; Pediatrics; Pulmonary medicine; Respiratory system.
© 2020 The Author(s).
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

