The potential of drug repositioning as a short-term strategy for the control and treatment of COVID-19 (SARS-CoV-2): a systematic review
- PMID: 32514689
- PMCID: PMC7276657
- DOI: 10.1007/s00705-020-04693-5
The potential of drug repositioning as a short-term strategy for the control and treatment of COVID-19 (SARS-CoV-2): a systematic review
Abstract
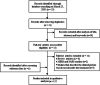
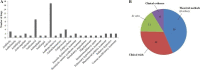
The novel human coronavirus (SARS-CoV-2), the causative agent of COVID-19, has quickly become a threat to the public health and economy worldwide. Despite the severity of some cases, there are no current pathogen-specific antivirals available to treat the disease. Therefore, many studies have focused on the evaluation of the anti-SARS-CoV-2 activity of clinically available drugs. Here, we conducted a systematic review to describe the drug repositioning strategy against SARS-CoV-2 and to discuss the clinical impact of this approach in the current pandemic context. The systematic review was performed on March 23, 2020, using PubMed/MEDLINE, Scopus, Cochrane Library, and Biblioteca Virtual de Saúde (BVS). The data were summarized in tables and critically analyzed. After the database search, 12 relevant studies were identified as eligible for the review. Among the drugs reported in these studies, 57 showed some evidence of antiviral activity. Antivirals, especially antiretrovirals, are the main class of therapeutic agents evaluated against COVID-19. Moreover, studies have reported the anti-SARS-CoV-2 activity of antitumor (16%; 9/57), antimalarial (7%, 4/57), and antibacterial (5%; 3/57) agents. Additionally, seven pharmacological agents (chloroquine, tetrandrine, umifenovir (arbidol), carrimycin, damageprevir, lopinavir/ritonavir) are in phase IV of clinical trials. Due to the evidence of the anti-SARS-CoV-2 activity of various clinically available agents, drug repositioning stands out as a promising strategy for a short-term response in the fight against the novel coronavirus.
Conflict of interest statement
All authors report that they do not have any conflicts of interest.
Figures
References
-
- de Wilde AH, Snijder EJ, Kikkert M, van Hemert MJ. Host factors in coronavirus replication. In: Ahmed R, Akira S, Aktories K, Casadevall A, Compans RW, Galan JE, Garcia-Sastre A, Malissen B, Rappuoli R, editors. Current topics in microbiology and immunology. 3. Switzerland: Springer Verlag; 2018. pp. 1–42. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

