How Often Does Modern Neoadjuvant Chemotherapy Downstage Patients to Breast-Conserving Surgery?
- PMID: 32514804
- PMCID: PMC7722183
- DOI: 10.1245/s10434-020-08593-5
How Often Does Modern Neoadjuvant Chemotherapy Downstage Patients to Breast-Conserving Surgery?
Abstract
Background: Neoadjuvant chemotherapy (NAC) has been proven to increase breast-conserving surgery (BCS) rates, but data are limited on conversion rates from BCS-ineligible (BCSi) to BCS-eligible (BCSe), specifically, in patients with large tumors.
Methods: Consecutive patients with stage I-III breast cancer treated with NAC from November 2013 to March 2019 were identified. BCS eligibility before and after NAC was prospectively determined. Patients deemed BCSi before NAC due to large tumor size were studied. Statistical analyses were conducted using Student's t-test, Wilcoxon rank sum test, Chi-square test, Fisher's test, and logistic regression.
Results: In this study, 600 of 1353 cancers were BCSi with large tumors; 69% were non-BCS candidates, 31% were borderline-BCS (bBCS) candidates. Of non-BCS candidates, 69% became BCSe after NAC; 66% chose BCS, and 90% were successful. Among bBCS candidates, 87% were BCSe after NAC, 73% chose BCS, and 96% were successful. On univariate analysis, bBCS candidacy, lower cT stage, cN0 status, absence of calcifications, human epidermal growth factor receptor 2 positive (HER2+)/triple negative (TN) receptor status, poor differentiation, ductal histology, and breast pCR were associated with conversion to BCS eligibility. On multivariable analysis, receptor status (hormone receptor positive [HR+]/HER2- ref; odds ratio [OR] HER2+ 1.63, P = 0.047; HR-/HER2- OR, 2.26, P = 0.003) and breast pCR (OR 2.62, P < 0.001) predicted successful downstaging, while larger clinical tumor size (OR 0.86, P = 0.003), non-BCS candidacy (OR 0.46, P = 0.003), cN+ status (OR 0.54, P = 0.008), and calcifications (OR 0.56, P = 0.007) predicted lower downstaging rates.
Conclusion: In patients with large tumors precluding BCS, conversion to BCS eligibility was high with NAC, particularly in bBCS candidates. HER2+/TN receptor status predicted successful downstaging, while lower downstaging rates were observed with larger tumors, cN+ status, and calcifications. These factors should be considered when selecting patients for NAC.
Figures

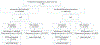
References
-
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. February 10 2008;26(5):778–785. - PubMed
-
- Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. October 2014;260(4):608–614; discussion 614–606. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

