Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury
- PMID: 32515141
- PMCID: PMC7348165
- DOI: 10.1111/jcmm.15478
Thermosensitive heparin-poloxamer hydrogel encapsulated bFGF and NGF to treat spinal cord injury
Abstract
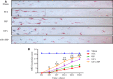
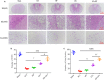
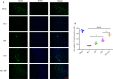
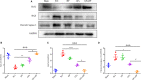
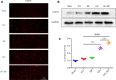
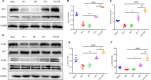
The application of growth factors (GFs) for treating chronic spinal cord injury (SCI) has been shown to promote axonal regeneration and functional recovery. However, direct administration of GFs is limited by their rapid degradation and dilution at the injured sites. Moreover, SCI recovery is a multifactorial process that requires multiple GFs to participate in tissue regeneration. Based on these facts, controlled delivery of multiple growth factors (GFs) to lesion areas is becoming an attractive strategy for repairing SCI. Presently, we developed a GFs-based delivery system (called GFs-HP) that consisted of basic fibroblast growth factor (bFGF), nerve growth factor (NGF) and heparin-poloxamer (HP) hydrogel through self-assembly mode. This GFs-HP was a kind of thermosensitive hydrogel that was suitable for orthotopic administration in vivo. Meanwhile, a 3D porous structure of this hydrogel is commonly used to load large amounts of GFs. After single injection of GFs-HP into the lesioned spinal cord, the sustained release of NGF and bFGF from HP could significantly improve neuronal survival, axon regeneration, reactive astrogliosis suppression and locomotor recovery, when compared with the treatment of free GFs or HP. Moreover, we also revealed that these neuroprotective and neuroregenerative effects of GFs-HP were likely through activating the phosphatidylinositol 3 kinase and protein kinase B (PI3K/Akt) and mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signalling pathways. Overall, our work will provide an effective therapeutic strategy for SCI repair.
Keywords: basic fibroblast growth factor (bFGF); heparin-poloxamer (HP) hydrogel; nerve growth factor (NGF); neuroprotection; spinal cord injury.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that this article has no conflicts of interest.
Figures


References
-
- Witiw CD, Fehlings MG. Acute spinal cord injury. J Spinal Disord Tech. 2015;28:202‐210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

