Metabolite cycled liver 1 H MRS on a 7 T parallel transmit system
- PMID: 32515151
- PMCID: PMC7379278
- DOI: 10.1002/nbm.4343
Metabolite cycled liver 1 H MRS on a 7 T parallel transmit system
Abstract
Introduction: Single-voxel 1 H MRS in body applications often suffers from respiratory and other motion induced phase and frequency shifts, which lead to incoherent averaging and hence to suboptimal results.
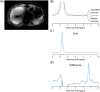
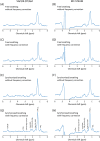
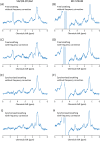
Methods: Here we show the application of metabolite cycling (MC) for liver STEAM-localized 1 H MRS on a 7 T parallel transmit system, using eight transmit-receive fractionated dipole antennas with 16 additional, integrated receive loops. MC-STEAM measurements were made in six healthy, lean subjects and compared with STEAM measurements using VAPOR water suppression. Measurements were performed during free breathing and during synchronized breathing, for which the subjects did breathe in between the MRS acquisitions. Both intra-session repeatability and inter-session reproducibility of liver lipid quantification with MC-STEAM and VAPOR-STEAM were determined.
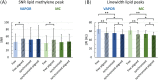
Results: The preserved water signal in MC-STEAM allowed for robust phase and frequency correction of individual acquisitions before averaging, which resulted in in vivo liver spectra that were of equal quality when measurements were made with free breathing or synchronized breathing. Intra-session repeatability and inter-session reproducibility of liver lipid quantification were better for MC-STEAM than for VAPOR-STEAM. This may also be explained by the more robust phase and frequency correction of the individual MC-STEAM acquisitions as compared with the VAPOR-STEAM acquisitions, for which the low-signal-to-noise ratio lipid signals had to be used for the corrections.
Conclusion: Non-water-suppressed MC-STEAM on a 7 T system with parallel transmit is a promising approach for 1 H MRS applications in the body that are affected by motion, such as in the liver, and yields better repeatability and reproducibility compared with water-suppressed measurements.
Keywords: 7 T; MRS; lipid composition; lipids; liver; metabolite cycling; parallel transmit; ultra-high field.
© 2020 The Authors. NMR in Biomedicine published by John Wiley & Sons Ltd.
Figures


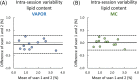
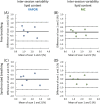
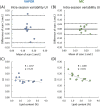
References
-
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221‐1231. - PubMed
-
- Araya J, Rodrigo R, Videla LA, et al. Increase in long‐chain polyunsaturated fatty acid n − 6/n − 3 ratio in relation to hepatic steatosis in patients with non‐alcoholic fatty liver disease. Clin Sci. 2004;106(6):635‐643. - PubMed
-
- Elizondo A, Araya J, Rodrigo R, et al. Polyunsaturated fatty acid pattern in liver and erythrocyte phospholipids from obese patients. Obesity. 2007;15(1):24‐31. - PubMed
-
- Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081‐1090. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

