Delineating the early transcriptional specification of the mammalian trachea and esophagus
- PMID: 32515350
- PMCID: PMC7282815
- DOI: 10.7554/eLife.55526
Delineating the early transcriptional specification of the mammalian trachea and esophagus
Abstract
The genome-scale transcriptional programs that specify the mammalian trachea and esophagus are unknown. Though NKX2-1 and SOX2 are hypothesized to be co-repressive master regulators of tracheoesophageal fates, this is untested at a whole transcriptomic scale and their downstream networks remain unidentified. By combining single-cell RNA-sequencing with bulk RNA-sequencing of Nkx2-1 mutants and NKX2-1 ChIP-sequencing in mouse embryos, we delineate the NKX2-1 transcriptional program in tracheoesophageal specification, and discover that the majority of the tracheal and esophageal transcriptome is NKX2-1 independent. To decouple the NKX2-1 transcriptional program from regulation by SOX2, we interrogate the expression of newly-identified tracheal and esophageal markers in Sox2/Nkx2-1 compound mutants. Finally, we discover that NKX2-1 binds directly to Shh and Wnt7b and regulates their expression to control mesenchymal specification to cartilage and smooth muscle, coupling epithelial identity with mesenchymal specification. These findings create a new framework for understanding early tracheoesophageal fate specification at the genome-wide level.
Keywords: Nkx2-1; Sox2; developmental biology; esophagus; genetics; genomics; lung; mouse; trachea; tracheoesophageal fistula.
Plain language summary
The trachea or windpipe is a tube that connects the throat to the lungs, while the esophagus connects the throat to the stomach. The trachea has cartilage rings that help to ensure clear airflow to the lungs, while the esophagus walls are lined with muscles that help to move food to the stomach. Although there are many differences between them, both the trachea and esophagus form from the same group of cells during development. Proteins called transcription factors help to control the formation of different body parts by switching different groups of genes on and off in different subsets of cells. Existing research has suggested that a transcription factor called NKX2.1 drives trachea formation, while another, called SOX2, is important in esophagus formation. An absence of either of these two proteins is thought to be associated with serious birth defects including loss of the trachea or esophagus, or failure of the two to separate fully. How these two transcription factors interact and drive the development of the trachea and esophagus, however, is currently unclear. Kuwahara et al. used mice to study the role of NKX2.1 and SOX2 in the formation of the trachea and esophagus. The findings identify many new genes that are active in the trachea and esophagus and reveal that NKX2.1 is not a master regulator that controls all of the genes involved in trachea formation. However, NKX2.1 does control several key genes, particularly those involved in forming cartilage in the trachea instead of muscle in the esophagus. The investigation also revealed many genes that are not controlled by NKX2.1 suggesting that other, currently unknown, systems play a major role in trachea formation. More work is required to understand the wider genetic regulators involved in differentiating the trachea from the esophagus. The findings in this study will help researchers to understand birth defects in the trachea and esophagus that result from genetic errors. They also reveal information about gene regulation processes that are relevant to the formation of other body parts and in the context of other diseases. In the long term, they could support regenerative medicine to regrow or replace lost or damaged body parts using lab-grown stem cells.
© 2020, Kuwahara et al.
Conflict of interest statement
AK, AL, CC, FL, MP, JB No competing interests declared
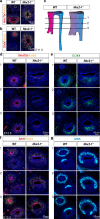
Figures


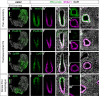






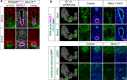

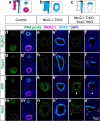
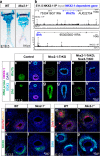

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

