An ECF-type transporter scavenges heme to overcome iron-limitation in Staphylococcus lugdunensis
- PMID: 32515736
- PMCID: PMC7299338
- DOI: 10.7554/eLife.57322
An ECF-type transporter scavenges heme to overcome iron-limitation in Staphylococcus lugdunensis
Abstract
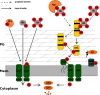
Energy-coupling factor type transporters (ECF) represent trace nutrient acquisition systems. Substrate binding components of ECF-transporters are membrane proteins with extraordinary affinity, allowing them to scavenge trace amounts of ligand. A number of molecules have been described as substrates of ECF-transporters, but an involvement in iron-acquisition is unknown. Host-induced iron limitation during infection represents an effective mechanism to limit bacterial proliferation. We identified the iron-regulated ECF-transporter Lha in the opportunistic bacterial pathogen Staphylococcus lugdunensis and show that the transporter is specific for heme. The recombinant substrate-specific subunit LhaS accepted heme from diverse host-derived hemoproteins. Using isogenic mutants and recombinant expression of Lha, we demonstrate that its function is independent of the canonical heme acquisition system Isd and allows proliferation on human cells as sources of nutrient iron. Our findings reveal a unique strategy of nutritional heme acquisition and provide the first example of an ECF-transporter involved in overcoming host-induced nutritional limitation.
Keywords: ECF-transporter; heme; infectious disease; iron; microbiology; nutritional immunity; staphylococcus lugdunensis.
© 2020, Jochim et al.
Conflict of interest statement
AJ, LA, DB, NS, IS, DC, SM, PV, DH, DS, SH No competing interests declared
Figures





References
-
- Beasley FC, Marolda CL, Cheung J, Buac S, Heinrichs DE. Staphylococcus aureus transporters hts, sir, and sst capture iron liberated from human transferrin by staphyloferrin A, staphyloferrin B, and catecholamine stress hormones, Respectively, and contribute to virulence. Infection and Immunity. 2011;79:2345–2355. doi: 10.1128/IAI.00117-11. - DOI - PMC - PubMed
-
- Bowden CFM, Chan ACK, Li EJW, Arrieta AL, Eltis LD, Murphy MEP. Structure-function analyses reveal key features in Staphylococcus aureus IsdB-associated unfolding of the heme-binding pocket of human hemoglobin. Journal of Biological Chemistry. 2018;293:177–190. doi: 10.1074/jbc.M117.806562. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

