Implementation of Germline Testing for Prostate Cancer: Philadelphia Prostate Cancer Consensus Conference 2019
- PMID: 32516092
- PMCID: PMC7430215
- DOI: 10.1200/JCO.20.00046
Implementation of Germline Testing for Prostate Cancer: Philadelphia Prostate Cancer Consensus Conference 2019
Abstract
Purpose: Germline testing (GT) is a central feature of prostate cancer (PCA) treatment, management, and hereditary cancer assessment. Critical needs include optimized multigene testing strategies that incorporate evolving genetic data, consistency in GT indications and management, and alternate genetic evaluation models that address the rising demand for genetic services.
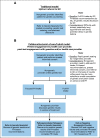
Methods: A multidisciplinary consensus conference that included experts, stakeholders, and national organization leaders was convened in response to current practice challenges and to develop a genetic implementation framework. Evidence review informed questions using the modified Delphi model. The final framework included criteria with strong (> 75%) agreement (Recommend) or moderate (50% to 74%) agreement (Consider).
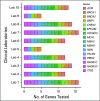
Results: Large germline panels and somatic testing were recommended for metastatic PCA. Reflex testing-initial testing of priority genes followed by expanded testing-was suggested for multiple scenarios. Metastatic disease or family history suggestive of hereditary PCA was recommended for GT. Additional family history and pathologic criteria garnered moderate consensus. Priority genes to test for metastatic disease treatment included BRCA2, BRCA1, and mismatch repair genes, with broader testing, such as ATM, for clinical trial eligibility. BRCA2 was recommended for active surveillance discussions. Screening starting at age 40 years or 10 years before the youngest PCA diagnosis in a family was recommended for BRCA2 carriers, with consideration in HOXB13, BRCA1, ATM, and mismatch repair carriers. Collaborative (point-of-care) evaluation models between health care and genetic providers was endorsed to address the genetic counseling shortage. The genetic evaluation framework included optimal pretest informed consent, post-test discussion, cascade testing, and technology-based approaches.
Conclusion: This multidisciplinary, consensus-driven PCA genetic implementation framework provides novel guidance to clinicians and patients tailored to the precision era. Multiple research, education, and policy needs remain of importance.
Figures

Comment in
-
Foreword: Prostate Cancer Genetics: The Urologic Research Promissory Note Is Being Cashed.Urol Clin North Am. 2021 Aug;48(3):xi-xii. doi: 10.1016/j.ucl.2021.06.002. Urol Clin North Am. 2021. PMID: 34210496 No abstract available.
References
-
- Giri VN, Hyatt C, Gomella LG. GT for men with prostate cancer: Navigating an expanding new world of genetic evaluation for precision therapy and precision management. J Clin Oncol. 2019;37:1455–1459. - PubMed
-
- Cheng HH, Sokolova AO, Schaeffer EM, et al. Germline and somatic mutations in prostate cancer for the clinician. J Natl Compr Canc Netw. 2019;17:515–521. - PubMed
-
- National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Prostate cancer (version 4.2019) https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf - PubMed
-
- Morgans AK, Szymaniak BM. Genetically-informed treatment for advanced and metastatic prostate cancer. Can J Urol. 2019;26:54–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

