Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice
- PMID: 32516134
- PMCID: PMC7524482
- DOI: 10.1172/JCI138697
Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice
Abstract
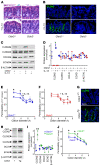
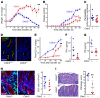
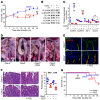
The tight junction protein claudin-2 is upregulated in disease. Although many studies have linked intestinal barrier loss to local and systemic disease, these have relied on macromolecular probes. In vitro analyses show, however, that these probes cannot be accommodated by size- and charge-selective claudin-2 channels. We sought to define the impact of claudin-2 channels on disease. Transgenic claudin-2 overexpression or IL-13-induced claudin-2 upregulation increased intestinal small cation permeability in vivo. IL-13 did not, however, affect permeability in claudin-2-knockout mice. Claudin-2 is therefore necessary and sufficient to effect size- and charge-selective permeability increases in vivo. In chronic disease, T cell transfer colitis severity was augmented or diminished in claudin-2-transgenic or -knockout mice, respectively. We translated the in vitro observation that casein kinase-2 (CK2) inhibition blocks claudin-2 channel function to prevent acute, IL-13-induced, claudin-2-mediated permeability increases in vivo. In chronic immune-mediated colitis, CK2 inhibition attenuated progression in claudin-2-sufficient, but not claudin-2-knockout, mice, i.e., the effect was claudin-2 dependent. Paracellular flux mediated by claudin-2 channels can therefore promote immune-mediated colitis progression. Although the mechanisms by which claudin-2 channels intensify disease remain to be defined, these data suggest that claudin-2 may be an accessible target in immune-mediated disorders, including inflammatory bowel disease.
Keywords: Cell Biology; Epithelial transport of ions and water; Gastroenterology; Inflammatory bowel disease; Tight junctions.
Conflict of interest statement
Figures




Comment in
-
Claudin-2 pore causes leak that breaches the dam in intestinal inflammation.J Clin Invest. 2020 Oct 1;130(10):5100-5101. doi: 10.1172/JCI140528. J Clin Invest. 2020. PMID: 32831294 Free PMC article.
-
CBFB-MYH11 fusion neoantigen enables T cell recognition and killing of acute myeloid leukemia.J Clin Invest. 2020 Oct 1;130(10):5127-5141. doi: 10.1172/JCI137723. J Clin Invest. 2020. PMID: 32831296 Free PMC article.
References
-
- Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol. 1984;80(1):59–70. doi: 10.1007/BF01868690. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

