Baloxavir Marboxil Single-dose Treatment in Influenza-infected Children: A Randomized, Double-blind, Active Controlled Phase 3 Safety and Efficacy Trial (miniSTONE-2)
- PMID: 32516282
- PMCID: PMC7360097
- DOI: 10.1097/INF.0000000000002747
Baloxavir Marboxil Single-dose Treatment in Influenza-infected Children: A Randomized, Double-blind, Active Controlled Phase 3 Safety and Efficacy Trial (miniSTONE-2)
Abstract
Background: Baloxavir marboxil (baloxavir) is a novel, cap-dependent endonuclease inhibitor that has previously demonstrated efficacy in the treatment of influenza in adults and adolescents. We assessed the safety and efficacy of baloxavir in otherwise healthy children with acute influenza.
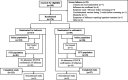
Methods: MiniSTONE-2 (Clinicaltrials.gov: NCT03629184) was a double-blind, randomized, active controlled trial enrolling children 1-<12 years old with a clinical diagnosis of influenza. Children were randomized 2:1 to receive either a single dose of oral baloxavir or oral oseltamivir twice daily for 5 days. The primary endpoint was incidence, severity and timing of adverse events (AEs); efficacy was a secondary endpoint.
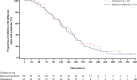
Results: In total, 173 children were randomized and dosed, 115 to the baloxavir group and 58 to the oseltamivir group. Characteristics of participants were similar between treatment groups. Overall, 122 AEs were reported in 84 (48.6%) children. Incidence of AEs was similar between baloxavir and oseltamivir groups (46.1% vs. 53.4%, respectively). The most common AEs were gastrointestinal (vomiting/diarrhea) in both groups [baloxavir: 12 children (10.4%); oseltamivir: 10 children (17.2%)]. No deaths, serious AEs or hospitalizations were reported. Median time (95% confidence interval) to alleviation of signs and symptoms of influenza was similar between groups: 138.1 (116.6-163.2) hours with baloxavir versus 150.0 (115.0-165.7) hours with oseltamivir.
Conclusions: Oral baloxavir is well tolerated and effective at alleviating symptoms in otherwise healthy children with acute influenza. Baloxavir provides a new therapeutic option with a simple oral dosing regimen.
Figures
References
-
- World Health Organization. Influenza (seasonal) fact sheet. 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Accessed January 13, 2020.
-
- Rotrosen ET, Neuzil KM.Influenza: a global perspective. Pediatr Clin North Am. 2017;64:911–936. - PubMed
-
- Paules C, Subbarao K.Influenza. Lancet. 2017;390:697–708. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

