Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas
- PMID: 32516388
- PMCID: PMC7523451
- DOI: 10.1093/neuonc/noaa141
Consensus recommendations for a dynamic susceptibility contrast MRI protocol for use in high-grade gliomas
Abstract
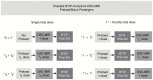
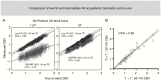
Despite the widespread clinical use of dynamic susceptibility contrast (DSC) MRI, DSC-MRI methodology has not been standardized, hindering its utilization for response assessment in multicenter trials. Recently, the DSC-MRI Standardization Subcommittee of the Jumpstarting Brain Tumor Drug Development Coalition issued an updated consensus DSC-MRI protocol compatible with the standardized brain tumor imaging protocol (BTIP) for high-grade gliomas that is increasingly used in the clinical setting and is the default MRI protocol for the National Clinical Trials Network. After reviewing the basis for controversy over DSC-MRI protocols, this paper provides evidence-based best practices for clinical DSC-MRI as determined by the Committee, including pulse sequence (gradient echo vs spin echo), BTIP-compliant contrast agent dosing (preload and bolus), flip angle (FA), echo time (TE), and post-processing leakage correction. In summary, full-dose preload, full-dose bolus dosing using intermediate (60°) FA and field strength-dependent TE (40-50 ms at 1.5 T, 20-35 ms at 3 T) provides overall best accuracy and precision for cerebral blood volume estimates. When single-dose contrast agent usage is desired, no-preload, full-dose bolus dosing using low FA (30°) and field strength-dependent TE provides excellent performance, with reduced contrast agent usage and elimination of potential systematic errors introduced by variations in preload dose and incubation time.
Keywords: DSC-MRI; cerebral blood volume; clinical trial; consensus protocol; high-grade glioma.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14(2):249–265. - PubMed
-
- Belliveau JW, Kennedy DN Jr, McKinstry RC, et al. . Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254(5032):716–719. - PubMed
-
- Aronen HJ, Gazit IE, Louis DN, et al. . Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology. 1994; 191(1):41–51. - PubMed
-
- Maeda M, Itoh S, Kimura H, et al. . Tumor vascularity in the brain: evaluation with dynamic susceptibility-contrast MR imaging. Radiology. 1993; 189(1):233–238. - PubMed
-
- Donahue KM, Krouwer HG, Rand SD, et al. . Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43(6):845–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1P50CA211015-01A1/CA/NCI NIH HHS/United States
- R01 CA158079/CA/NCI NIH HHS/United States
- R21 CA223757/CA/NCI NIH HHS/United States
- R01 CA082500/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- R01 CA221938/CA/NCI NIH HHS/United States
- EP-C-15-003/EPA/EPA/United States
- R01 CA213158/CA/NCI NIH HHS/United States
- U01 CA176110/CA/NCI NIH HHS/United States
- R01 CA213158-01/CA/NCI NIH HHS/United States
- U01 CA220378/CA/NCI NIH HHS/United States
- P50 CA211015/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States

