Role of TH17 Responses in Increasing Herpetic Keratitis in the Eyes of Mice Infected with HSV-1
- PMID: 32516406
- PMCID: PMC7415293
- DOI: 10.1167/iovs.61.6.20
Role of TH17 Responses in Increasing Herpetic Keratitis in the Eyes of Mice Infected with HSV-1
Abstract
Purpose: TH17 cells play an important role in host defense and autoimmunity yet very little is known about the role of IL17 in herpes simplex virus (HSV)-1 infectivity. To better understand the relationship between IL17 and HSV-1 infection, we assessed the relative impact of IL17A-deficiency and deficiency of its receptors on HSV-1 responses in vivo.
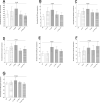
Methods: We generated IL17RA-/- and IL17RA-/-RC-/- mice in-house and infected them along with IL17A-/- and IL17RC-/- mice in the eyes with 2 × 105 PFU/eye of wild type (WT) HSV-1 strain McKrae. WT C57BL/6 mice were used as control. Virus replication in the eye, survival, corneal scarring (CS), angiogenesis, levels of latency-reactivation, and levels of CD8 and exhaustion markers (PD1, TIM3, LAG3, CTLA4, CD244, and CD39) in the trigeminal ganglia (TG) of infected mice were determined on day 28 postinfection.
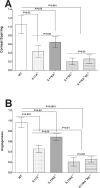
Results: No significant differences in virus replication in the eye, survival, latency, reactivation, and exhaustion markers were detected among IL17A-/-, IL17RA-/-, IL17RC-/-, IL17RA-/-RC-/-, and WT mice. However, mice lacking IL17 had significantly less CS and angiogenesis than WT mice. In addition, angiogenesis levels in the absence of IL17RC and irrespective of the absence of IL17RA were significantly less than in IL17A- or IL17RA-deficient mice.
Conclusions: Our results suggest that the absence of IL17 protects against HSV-1-induced eye disease, but has no role in protecting against virus replication, latency, or reactivation. In addition, our data provide rationale for blocking IL17RC function rather than IL17A or IL17RA function as a key driver of HSV-1-induced eye disease.
Conflict of interest statement
Disclosure:
Figures




References
-
- Sad S, Li L, Mosmann TR. Cytokine-deficient CD8+ Tc1 cells induced by IL-4: retained inflammation and perforin and Fas cytotoxicity but compromised long term killing of tumor cells. J Immunol. 1997; 159: 606–613. - PubMed
-
- Mosmann TR, Yokota T, Kastelein R, Zurawski SM, Arai N, Takebe Y. Species-specificity of T cell stimulating activities of IL 2 and BSF-1 (IL 4): comparison of normal and recombinant, mouse and human IL 2 and BSF-1 (IL 4). J Immunol. 1987; 138: 1813–1816. - PubMed
-
- Street NE, Schumacher JH, Fong TA, et al. .. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990; 144: 1629–1639. - PubMed
-
- Schmitt E, Klein M, Bopp T. Th9 cells, new players in adaptive immunity. Trends Immunol. 2014; 35: 61–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

