The centromere comes into focus: from CENP-A nucleosomes to kinetochore connections with the spindle
- PMID: 32516549
- PMCID: PMC7333888
- DOI: 10.1098/rsob.200051
The centromere comes into focus: from CENP-A nucleosomes to kinetochore connections with the spindle
Abstract
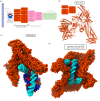
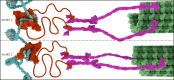
Eukaryotic chromosome segregation relies upon specific connections from DNA to the microtubule-based spindle that forms at cell division. The chromosomal locus that directs this process is the centromere, where a structure called the kinetochore forms upon entry into mitosis. Recent crystallography and single-particle electron microscopy have provided unprecedented high-resolution views of the molecular complexes involved in this process. The centromere is epigenetically specified by nucleosomes harbouring a histone H3 variant, CENP-A, and we review recent progress on how it differentiates centromeric chromatin from the rest of the chromosome, the biochemical pathway that mediates its assembly and how two non-histone components of the centromere specifically recognize CENP-A nucleosomes. The core centromeric nucleosome complex (CCNC) is required to recruit a 16-subunit complex termed the constitutive centromere associated network (CCAN), and we highlight recent structures reported of the budding yeast CCAN. Finally, the structures of multiple modular sub-complexes of the kinetochore have been solved at near-atomic resolution, providing insight into how connections are made to the CCAN on one end and to the spindle microtubules on the other. One can now build molecular models from the DNA through to the physical connections to microtubules.
Keywords: centromere; chromatin; epigenetics; kinetochore; mitosis; nucleosome.
Conflict of interest statement
We declare we have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

