Mutations in ASPRV1 Cause Dominantly Inherited Ichthyosis
- PMID: 32516568
- PMCID: PMC7332602
- DOI: 10.1016/j.ajhg.2020.05.013
Mutations in ASPRV1 Cause Dominantly Inherited Ichthyosis
Abstract
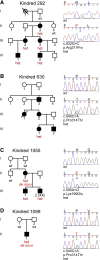
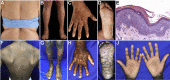
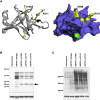
The discovery of genetic causes of inherited skin disorders has been pivotal to the understanding of epidermal differentiation, function, and renewal. Here we show via exome sequencing that mutations in ASPRV1 (aspartic peptidase retroviral-like 1) cause a dominant Mendelian disorder featuring palmoplantar keratoderma and lamellar ichthyosis, a phenotype that has otherwise been exclusively recessive. ASPRV1 encodes a mammalian-specific and stratified epithelia-specific protease important in processing of filaggrin, a critical component of the uppermost epidermal layer. Three different heterozygous ASPRV1 missense mutations in four unrelated ichthyosis kindreds segregate with disease and disrupt protein residues within close proximity to each other and autocatalytic cleavage sites. Expression of mutant ASPRV1 proteins demonstrates that all three mutations alter ASPRV1 auto-cleavage and filaggrin processing, a function vital to epidermal barrier integrity.
Keywords: ASPRV1; Mendelian; SASPase; de novo; dominant; epidermis; exome; ichthyosis; keratoderma; skin.
Copyright © 2020 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Traupe H., Kolde G., Happle R. Autosomal dominant lamellar ichthyosis: a new skin disorder. Clin. Genet. 1984;26:457–461. - PubMed
-
- Kolde G., Happle R., Traupe H. Autosomal-dominant lamellar ichthyosis: ultrastructural characteristics of a new type of congenital ichthyosis. Arch. Dermatol. Res. 1985;278:1–5. - PubMed
-
- Bernard D., Méhul B., Thomas-Collignon A., Delattre C., Donovan M., Schmidt R. Identification and characterization of a novel retroviral-like aspartic protease specifically expressed in human epidermis. J. Invest. Dermatol. 2005;125:278–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

