Using eHealth to Support COVID-19 Education, Self-Assessment, and Symptom Monitoring in the Netherlands: Observational Study
- PMID: 32516750
- PMCID: PMC7313382
- DOI: 10.2196/19822
Using eHealth to Support COVID-19 Education, Self-Assessment, and Symptom Monitoring in the Netherlands: Observational Study
Abstract
Background: The coronavirus disease (COVID-19) situation demands a lot from citizens, health care providers, and governmental institutions. Citizens need to cope with guidelines on social interaction, work, home isolation, and symptom recognition. Additionally, health care providers and policy makers have to cope with unprecedented and unpredictable pressure on the health care system they need to manage. By providing citizens with an app, they always have access to the latest information and can assess their own health. This data could be used to support policy makers and health care providers to get valuable insights in the regional distribution of infection load and health care consumption.
Objective: The aim of this observational study is to assess people's use of an app to support them with COVID-19 education, self-assessment, and monitoring of their own health for a 7-day period. In addition, we aim to assess the usability of this data for health care providers and policy makers by applying it to an interactive map and combining it with hospital data. The secondary outcomes of the study were user's satisfaction with the information provided in the app, perceived usefulness of the app, health care providers they contacted, and the follow-up actions from this contact.
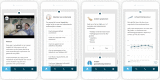
Methods: This observational cohort study was carried out at the nonacademic teaching hospital "Elisabeth Twee Steden" (ETZ) in Tilburg, Netherlands. From April 1, 2020, onwards ETZ offered the COVID-19 education, self-assessment, and symptom tracking diary to their already existing app for patient education and monitoring.
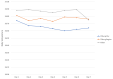
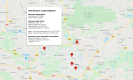
Results: Between April 1 and April 20, 2020, a total of 6194 people downloaded the app. The self-assessment functionality was used abundantly to check one's health status. In total, 5104 people responded to the question about severe symptoms, from which 242 indicated to suffer from severe symptoms. A total of 4929 people responded to the question about mild symptoms, from which 3248 indicated to suffer from these. The data was successfully applied to an interactive map, displaying user demographics and health status. Furthermore, the data was linked to clinical data. App users were satisfied with the information in the app and appreciated the symptom diary functionality. In total, 102 users reached out to a health care provider, leading to 91 contacts.
Conclusions: Our study demonstrated the successful implementation and use of an app with COVID-19 education, self-assessment, and a 7-day symptom diary. Data collected with the app were successfully applied to an interactive map. In addition, we were able to link the data to COVID-19 screening results from the hospital's microbiology laboratory. This data could be used to support policy makers and health care providers to get valuable insights in the regional distribution of infection load and health care consumption.
Trial registration: Netherlands Trial Register NL8501; https://www.trialregister.nl/trial/8501.
Keywords: COVID-19; eHealth; mHealth; mobile phone; patient education; self-management; smartphone.
©Thomas Timmers, Loes Janssen, Joep Stohr, J L Murk, M A H Berrevoets. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 23.06.2020.
Conflict of interest statement
Conflicts of Interest: The principal investigator, TT, is one of the cofounders of Interactive Studios. Interactive Studios is the company that developed the app used in this study. Interactive Studios offered the app used in this study free of charge. The coauthors declare that the research was conducted in the absence of any other commercial or financial relationships that could be construed as a potential conflict of interest. Moreover, all authors have completed the International Committee of Medical Journal Editors’ uniform disclosure form and declare the following: no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
-
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 May;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. http://europepmc.org/abstract/MED/32087114 - DOI - PMC - PubMed
-
- Alderweireld CEA, Buiting AGM, Murk JLAN, Verweij JJ, Berrevoets MAH, van Kasteren MEE. COVID-19: patiënt nul in Nederland (Patient zero in The Netherlands) Nederlands Tijdschrift voor Geneeskunde (NTVG) 2020;164(164):1. - PubMed
-
- RIVM. 2020. Actuele informatie over het nieuwe coronavirus (COVID-19) https://www.rivm.nl/coronavirus-covid-19/actueel.
-
- RIVM. The Netherlands: Rijksinstituut voor Volksgezondheid; 2020. Maatregelen om verspreiding van het virus te voorkomen (translated: measures to prevent the virus from spreading) https://www.rivm.nl/coronavirus-covid-19/vragen-antwoorden.
-
- Zamberg I, Manzano S, Posfay-Barbe K, Windisch O, Agoritsas T, Schiffer E. A mobile health platform to disseminate validated institutional measurements during the COVID-19 outbreak: utilization-focused evaluation study. JMIR Public Health Surveill. 2020 Apr 14;6(2):e18668. doi: 10.2196/18668. https://publichealth.jmir.org/2020/2/e18668/ - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

