Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2
- PMID: 32516797
- PMCID: PMC7486271
- DOI: 10.1038/s41586-020-2423-5
Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2
Abstract
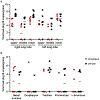
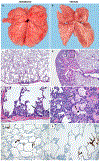
Effective therapies to treat coronavirus disease 2019 (COVID-19) are urgently needed. While many investigational, approved, and repurposed drugs have been suggested as potential treatments, preclinical data from animal models can guide the search for effective treatments by ruling out those that lack efficacy in vivo. Remdesivir (GS-5734) is a nucleotide analogue prodrug with broad antiviral activity1,2 that is currently being investigated in COVID-19 clinical trials and recently received Emergency Use Authorization from the US Food and Drug Administration3,4. In animal models, remdesivir was effective against infection with Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus (SARS-CoV)2,5,6. In vitro, remdesivir inhibited replication of SARS-CoV-27,8. Here we investigate the efficacy of remdesivir in a rhesus macaque model of SARS-CoV-2 infection9. Unlike vehicle-treated animals, macaques treated with remdesivir did not show signs of respiratory disease; they also showed reduced pulmonary infiltrates on radiographs and reduced virus titres in bronchoalveolar lavages twelve hours after the first dose. Virus shedding from the upper respiratory tract was not reduced by remdesivir treatment. At necropsy, remdesivir-treated animals had lower lung viral loads and reduced lung damage. Thus, treatment with remdesivir initiated early during infection had a clinical benefit in rhesus macaques infected with SARS-CoV-2. Although the rhesus macaque model does not represent the severe disease observed in some patients with COVID-19, our data support the early initiation of remdesivir treatment in patients with COVID-19 to prevent progression to pneumonia.
Conflict of interest statement
Conflict of interest
The authors affiliated with Gilead Sciences are employees of the company and own company stock. The authors affiliated with NIH have no conflict of interest to report.
Figures
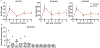
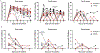



Update of
-
Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2.bioRxiv [Preprint]. 2020 Apr 22:2020.04.15.043166. doi: 10.1101/2020.04.15.043166. bioRxiv. 2020. Update in: Nature. 2020 Sep;585(7824):273-276. doi: 10.1038/s41586-020-2423-5. PMID: 32511319 Free PMC article. Updated. Preprint.
References
-
- Remdesivir. 2020. (Accessed 05/15/2020, at https://clinicaltrials.gov/ct2/results?cond=&term=remdesivir&cntry=&stat....)
-
- Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. 2020. (Accessed 05/15/2020, at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19....)
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

