BCMA-Targeting Therapy: Driving a New Era of Immunotherapy in Multiple Myeloma
- PMID: 32516895
- PMCID: PMC7352710
- DOI: 10.3390/cancers12061473
BCMA-Targeting Therapy: Driving a New Era of Immunotherapy in Multiple Myeloma
Abstract
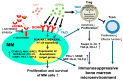
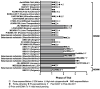
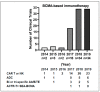
The treatment of multiple myeloma (MM) has entered into a new era of immunotherapy. Novel immunotherapies will significantly improve patient outcome via simultaneously targeting malignant plasma cell (PC) and reversing immunocompromised bone marrow (BM) microenvironment. B-cell maturation antigen (BCMA), selectively expressed in PCs and a key receptor for A proliferation-inducing ligand (APRIL), is highly expressed in MM cells from patients at all stages. The APRIL/BCMA signal cascades promote the survival and drug resistance of MM cells and further modulate immunosuppressive BM milieu. Impressively, anti-BCMA immunotherapeutic reagents, including chimeric antigen receptor (CAR), antibody-drug conjugate (ADC) and bispecific T cell engager (BiTE) have all shown high response rates in their first clinical trials in relapse and refractory patients with very limited treatment options. These results rapidly inspired numerous development of next-generation anti-BCMA biotherapeutics, i.e., bispecific molecule, bispecific or trispecific antibodies, a novel form of CAR T/NK cells and T Cell Antigen Coupler (TAC) receptors, antibody-coupled T cell receptor (ACTR) as well as a cancer vaccine. We here highlight seminal preclinical and clinical studies on novel BCMA-based immunotherapies as effective monotherapy and discuss their potential in combination with current anti-MM and novel checkpoint drugs in earlier disease stages to further achieve durable responses in patients.
Keywords: ADC; ADCC; ADCP; B-cell maturation antigen; BCMA; BM; BiTE; CAR T; MM; MoAb; NK cell; T cell dependent cytotoxicity; TDCC; antibody drug conjugate; antibody-dependent cellular cytotoxicity; antibody-dependent cellular phagocytosis; bispecific T cell engager; bone marrow; chimeric antigen receptor T cell; monoclonal antibody; multiple myeloma; natural killer cell; signal transduction; targeted immunotherapy; tumor targeting; tumor-associated antigen.
Conflict of interest statement
K.C.A. serves on advisory boards Celgene, Millennium-Takeda, Bristol-Myers Squibb, Gilead Sciences, Janssen, and Sanofi-Aventis and is a Scientific founder of OncoPep and C4 Therapeutics. All other authors declare no competing financial interests. All other authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

