Optimization of Protein Extraction Method for 2DE Proteomics of Goat's Milk
- PMID: 32516945
- PMCID: PMC7321142
- DOI: 10.3390/molecules25112625
Optimization of Protein Extraction Method for 2DE Proteomics of Goat's Milk
Abstract
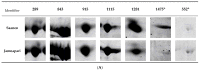
Two-dimensional electrophoretic (2DE)-based proteomics remains a powerful tool for allergenomic analysis of goat's milk but requires effective extraction of proteins to accurately profile the overall causative allergens. However, there are several current issues with goat's milk allergenomic analysis, and among these are the absence of established standardized extraction method for goat's milk proteomes and the complexity of goat's milk matrix that may hamper the efficacy of protein extraction. This study aimed to evaluate the efficacies of three different protein extraction methods, qualitatively and quantitatively, for the 2DE-proteomics, using milk from two commercial dairy goats in Malaysia, Saanen, and Jamnapari. Goat's milk samples from both breeds were extracted by using three different methods: a milk dilution in urea/thiourea based buffer (Method A), a triphasic separation protocol in methanol/chloroform solution (Method B), and a dilution in sulfite-based buffer (Method C). The efficacies of the extraction methods were assessed further by performing the protein concentration assay and 1D and 2D SDS-PAGE profiling, as well as identifying proteins by MALDI-TOF/TOF MS/MS. The results showed that method A recovered the highest amount of proteins (72.68% for Saanen and 71.25% for Jamnapari) and produced the highest number of protein spots (199 ± 16.1 and 267 ± 10.6 total spots for Saanen and Jamnapari, respectively) with superior gel resolution and minimal streaking. Six milk protein spots from both breeds were identified based on the positive peptide mass fingerprinting matches with ruminant milk proteins from public databases, using the Mascot software. These results attest to the fitness of the optimized protein extraction protocol, method A, for 2DE proteomic and future allergenomic analysis of the goat's milk.
Keywords: 2DE; gel electrophoresis; goat’s milk; protein extraction; proteomics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Monaci L., Tregoat V., Van Hengel A.J., Anklam E. Milk Allergens, Their Characteristics and Their Detection in Food: A Review. Eur. Food Res. Technol. 2006;223:149–179. doi: 10.1007/s00217-005-0178-8. - DOI
-
- Park Y.W. Hypo-Allergenic and Therapeutic Significance of Goat Milk. Small Rumin. Res. 1994;14:151–159. doi: 10.1016/0921-4488(94)90105-8. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

