Effect of Magnesium Supplementation on Circulating Biomarkers of Cardiovascular Disease
- PMID: 32517192
- PMCID: PMC7352673
- DOI: 10.3390/nu12061697
Effect of Magnesium Supplementation on Circulating Biomarkers of Cardiovascular Disease
Abstract
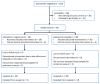
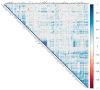
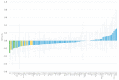
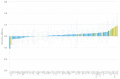
(1) Background: Magnesium supplementation may be effective for the prevention of cardiometabolic diseases, but the mechanisms are unclear. Proteomic approaches can assist in identifying the underlying mechanisms. (2) Methods: We collected repeated blood samples from 52 individuals enrolled in a double-blind trial which randomized participants 1:1 to oral magnesium supplementation (400 mg magnesium/day in the form of magnesium oxide) or a matching placebo for 10 weeks. Plasma levels of 91 proteins were measured at baseline with follow-up samples using the Olink Cardiovascular Disease III proximity extension assay panel and were modeled as arbitrary units in a log2 scale. We evaluated the effect of oral magnesium supplementation for changes in protein levels and the baseline association between serum magnesium and protein levels. The Holm procedure was used to adjust for multiple comparisons. (3) Results: Participants were 73% women, 94% white, and had a mean age of 62. Changes in proteins did not significantly differ between the two intervention groups after correction for multiple comparisons. The most statistically significant effects were on myoglobin [difference -0.319 log2 units, 95% confidence interval (CI) (-0.550, -0.088), p = 0.008], tartrate-resistant acid phosphatase type 5 (-0.187, (-0.328, -0.045), p = 0.011), tumor necrosis factor ligand superfamily member 13B (-0.181, (-0.332, -0.031), p = 0.019), ST2 protein (-0.198, (-0.363, -0.032), p = 0.020), and interleukin-1 receptor type 1 (-0.144, (-0.273, -0.015), p = 0.029). Similarly, none of the associations of baseline serum magnesium with protein levels were significant after correction for multiple comparisons. (4) Conclusions: Although we did not identify statistically significant effects of oral magnesium supplementation in this relatively small study, this study demonstrates the value of proteomic approaches for the investigation of mechanisms underlying the beneficial effects of magnesium supplementation. Clinical Trials Registration: ClinicalTrials.gov NCT02837328.
Keywords: magnesium; proteomics; randomized trial.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Del Gobbo L.C., Imamura F., Wu J.H., De Oliveira Otto M.C., Chiuve S.E., Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013;98:160–173. doi: 10.3945/ajcn.112.053132. - DOI - PMC - PubMed
-
- Misialek J.R., Lopez F.L., Lutsey P.L., Huxley R.R., Peacock J.M., Chen L.Y., Soliman E.Z., Agarwal S.K., Alonso A. Serum and dietary magnesium and incidence of atrial fibrillation in whites and in African Americans--Atherosclerosis Risk in Communities (ARIC) Study. Circ. J. 2013;77:323–329. doi: 10.1253/circj.CJ-12-0886. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

