Biological Factors behind Melanoma Response to Immune Checkpoint Inhibitors
- PMID: 32517213
- PMCID: PMC7313051
- DOI: 10.3390/ijms21114071
Biological Factors behind Melanoma Response to Immune Checkpoint Inhibitors
Abstract
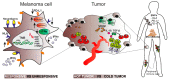
Modern immunotherapy together with targeted therapy has revolutionized the treatment of advanced melanoma. Inhibition of immune checkpoints significantly improved the median overall survival and gave hope to many melanoma patients. However, this treatment has three serious drawbacks: high cost, serious side effects, and an effectiveness limited only to approximately 50% of patients. Some patients do not derive any or short-term benefit from this treatment due to primary or secondary resistance. The response to immunotherapy depends on many factors that fall into three main categories: those associated with melanoma cells, those linked to a tumor and its microenvironment, and those classified as individual ontogenic and physiological features of the patient. The first category comprises expression of PD-L1 and HLA proteins on melanoma cells as well as genetic/genomic metrics such as mutational load, (de)activation of specific signaling pathways and epigenetic factors. The second category is the inflammatory status of the tumor: "hot" versus "cold" (i.e., high versus low infiltration of immune cells). The third category comprises metabolome and single nucleotide polymorphisms of specific genes. Here we present up-to-date data on those biological factors influencing melanoma response to immunotherapy with a special focus on signaling pathways regulating the complex process of anti-tumor immune response. We also discuss their potential predictive capacity.
Keywords: biomarkers of response; immune checkpoint inhibitors; immunotherapy; melanoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ugurel S., Rohmel J., Ascierto P.A., Flaherty K.T., Grob J.J., Hauschild A., Larkin J., Long G.V., Lorigan P., McArthur G.A., et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur. J. Cancer. 2017;83:247–257. doi: 10.1016/j.ejca.2017.06.028. - DOI - PubMed
-
- Da L.J., Teng Y.J., Wang N., Zaguirre K., Liu Y.T., Qi Y.L., Song F.X. Organ-Specific Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitor Monotherapy Versus Combination Therapy in Cancer: A Meta-Analysis of Randomized Controlled Trials. Front. Pharm. 2020;10 doi: 10.3389/fphar.2019.01671. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

