Sorption Mechanism and Optimization Study for the Bioremediation of Pb(II) and Cd(II) Contamination by Two Novel Isolated Strains Q3 and Q5 of Bacillus sp
- PMID: 32517236
- PMCID: PMC7312031
- DOI: 10.3390/ijerph17114059
Sorption Mechanism and Optimization Study for the Bioremediation of Pb(II) and Cd(II) Contamination by Two Novel Isolated Strains Q3 and Q5 of Bacillus sp
Abstract
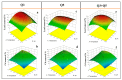
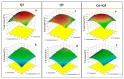
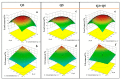
The use of bacterial strains as agents in bioremediation processes could reduce the harmfulness of potential toxic elements (PTEs) from water and soil with low or even no impact on the natural ecosystems. In this study, two new metal resistant-bacterial strains (Q3 and Q5) of Bacillus sp. were isolated from a sulfurous spring and their potential (as pure cultures or mixed) to remove Pb(II) and Cd(II) from an aqueous matrix was evaluated and optimized using response surface methodology (RSM). The optimal conditions for Cd(II) removal from all tested strains combinations were observed at an initial pH 5, a temperature of 38 °C, and an initial Cd(II) concentration of 50 mg L-1, while the performance of bacterial strains on Pb(II) removal was strongly correlated to initial pH and temperature conditions. Moreover, the efficiency of bacterial strains in removing both PTEs, Pb(II) and Cd(II), from an aqueous matrix was considerably higher when they were used as a mixed culture rather than pure. According to field emission SEM (FESEM) and EDS analysis, the two bacterial strains showed different mechanisms in removing Cd(II): Bacillus sp. Q5 bio-accumulated Cd(II) in its periplasmic space, whereas Bacillus sp. Q3 bio-accumulated Cd(II) on its cell surface. On the other hand, Pb(II) is removed by chemical precipitation (lead sulfide) induced by both Bacillus sp. Q3 and Q5. This study discloses new aspects of Pb(II) and Cd(II) bioremediation mechanisms in Bacillus species that can be extremely useful for designing and operating novel PTEs bioremediation processes.
Keywords: PTEs removal; Pb precipitation; SEM; bacterial strains; bioaccumulation; bioremediation; central composite design.
Conflict of interest statement
The author declares that there is no conflict of interest.
Figures







References
-
- Dabir A., Heidari P., Ghorbani H., Ebrahimi A. Cadmium and lead removal by new bacterial isolates from coal and aluminum mines. Int. J. Environ. Sci. Technol. 2019;16:8297–8304. doi: 10.1007/s13762-019-02303-9. - DOI
-
- Ferraro A., Fabbricino M., van Hullebusch E.D., Esposito G. Investigation of different ethylenediamine-N,N′-disuccinic acid-enhanced washing configurations for remediation of a Cu-contaminated soil: Process kinetics and efficiency comparison between single-stage and multi-stage configurations. Environ. Sci. Pollut. Res. 2017;24:21960–21972. doi: 10.1007/s11356-017-9844-1. - DOI - PubMed
-
- Bueno B.Y.M., Torem M.L., Molina F., de Mesquita L.M.S. Biosorption of lead(II), chromium(III) and copper(II) by R. opacus: Equilibrium and kinetic studies. Miner. Eng. 2008;21:65–75. doi: 10.1016/j.mineng.2007.08.013. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

