Propagation, Inactivation, and Safety Testing of SARS-CoV-2
- PMID: 32517266
- PMCID: PMC7354523
- DOI: 10.3390/v12060622
Propagation, Inactivation, and Safety Testing of SARS-CoV-2
Abstract
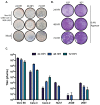
In late 2019, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, the capital of the Chinese province Hubei. Since then, SARS-CoV-2 has been responsible for a worldwide pandemic resulting in over 4 million infections and over 250,000 deaths. The pandemic has instigated widespread research related to SARS-CoV-2 and the disease that it causes, COVID-19. Research into this new virus will be facilitated by the availability of clearly described and effective procedures that enable the propagation and quantification of infectious virus. As work with the virus is recommended to be performed at biosafety level 3, validated methods to effectively inactivate the virus to enable the safe study of RNA, DNA, and protein from infected cells are also needed. Here, we report methods used to grow SARS-CoV-2 in multiple cell lines and to measure virus infectivity by plaque assay using either agarose or microcrystalline cellulose as an overlay as well as a SARS-CoV-2 specific focus forming assay. We also demonstrate effective inactivation by TRIzol, 10% neutral buffered formalin, beta propiolactone, and heat.
Keywords: SARS-CoV-2; coronavirus; inactivation; plaque assay; virology; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization . Situation Report—112 Coronavirus Disease 2019 (COVID-19) World Health Organization; Geneva, Switzerland: 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

