Multinucleated giant cell phenotype in response to stimulation
- PMID: 32517879
- PMCID: PMC7292728
- DOI: 10.1016/j.imbio.2020.151952
Multinucleated giant cell phenotype in response to stimulation
Abstract
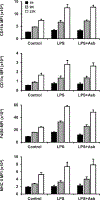
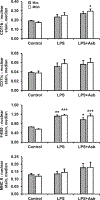
Macrophages fuse into multinucleated giant cells (MGC) in many pathological conditions. Despite MGC correlations with granulomas, their functional contribution to inflammation is relatively unknown. An in vitro mouse model of IL-4-induced bone marrow-derived macrophage fusion and microfiltration were used to generate enriched MGC and macrophage populations. Phenotypes were compared in response to well-known inflammatory stimuli, including lipopolysaccharide and crocidolite asbestos. Surface markers were assessed by flow cytometry: CD11b, CD11c, F4/80, and MHC II. Secreted cytokines were assessed by multiplex immunoassay: IFN-γ, IL-1β, IL-6, TNF-α, IL-10, IL-13, and IL-33. Results show that MGC maintained macrophage surface protein expression but lost the ability to produce a cytokine response. This suggests a potentially beneficial role of MGC in isolating the host from a foreign body without contributing to excessive inflammation. This study and future research using other stimulants and environments are important to gaining a fundamental MGC cell biology understanding. This will inform approaches to controlling the foreign body response to particle exposure, medical implants, and many diseases associated with granulomas.
Keywords: Cytokine; In vitro; Macrophage; Mouse; Multinucleated giant cell; Phenotype; Surface marker.
Copyright © 2020 Elsevier GmbH. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Factors influencing multinucleated giant cell formation in vitro.Immunobiology. 2019 Nov;224(6):834-842. doi: 10.1016/j.imbio.2019.08.002. Epub 2019 Aug 10. Immunobiology. 2019. PMID: 31439452 Free PMC article.
-
Differential effects of macrophage- and granulocyte-macrophage colony-stimulating factors on cytokine gene expression during rat alveolar macrophage differentiation into multinucleated giant cells (MGC): role for IL-6 in type 2 MGC formation.J Immunol. 1996 Dec 1;157(11):5118-25. J Immunol. 1996. PMID: 8943422
-
Differential regulation of formation of multinucleated giant cells from concanavalin A-stimulated human blood monocytes by IFN-gamma and IL-4.J Immunol. 1993 Apr 1;150(7):3002-10. J Immunol. 1993. PMID: 8454870
-
[Human monocyte-derived multinucleated giant cells].Nihon Hansenbyo Gakkai Zasshi. 2004 Sep;73(3):245-51. doi: 10.5025/hansen.73.245. Nihon Hansenbyo Gakkai Zasshi. 2004. PMID: 15508727 Review. Japanese.
-
Purinergic signaling in giant cell formation.Front Biosci (Elite Ed). 2012 Jan 1;4(1):41-55. doi: 10.2741/359. Front Biosci (Elite Ed). 2012. PMID: 22201854 Review.
Cited by
-
Biodegradation of HA and β-TCP Ceramics Regulated by T-Cells.Pharmaceutics. 2022 Sep 16;14(9):1962. doi: 10.3390/pharmaceutics14091962. Pharmaceutics. 2022. PMID: 36145710 Free PMC article.
-
The aging ovary stands on the shoulders of giant multinucleated cells.PLoS Biol. 2025 Jun 24;23(6):e3003216. doi: 10.1371/journal.pbio.3003216. eCollection 2025 Jun. PLoS Biol. 2025. PMID: 40554744 Free PMC article.
-
Cranial Mandibular Fibrosis Syndrome in Adult Farmed Rainbow Trout Oncorhynchus mykiss.Pathogens. 2021 Apr 30;10(5):542. doi: 10.3390/pathogens10050542. Pathogens. 2021. PMID: 33946332 Free PMC article.
-
Heterogeneity and Actin Cytoskeleton in Osteoclast and Macrophage Multinucleation.Int J Mol Sci. 2020 Sep 10;21(18):6629. doi: 10.3390/ijms21186629. Int J Mol Sci. 2020. PMID: 32927783 Free PMC article. Review.
-
Glucosylated cholesterol accumulates in atherosclerotic lesions and impacts macrophage immune response.J Lipid Res. 2025 Jun;66(6):100825. doi: 10.1016/j.jlr.2025.100825. Epub 2025 May 15. J Lipid Res. 2025. PMID: 40381699 Free PMC article.
References
-
- Brown TA, Holian A, Pinkerton KE, Lee JW, Cho YH, 2016. Early life exposure to environmental tobacco smoke alters immune response to asbestos via a shift in inflammatory phenotype resulting in increased disease development. Inhal Toxicol 28, 349–356. 10.1080/08958378.2016.1175526 - DOI - PMC - PubMed
-
- Davison AG, Haslam PL, Corrin B, Coutts II, Dewar A, Riding WD, Studdy PR, Newman-Taylor AJ, 1983. Interstitial lung disease and asthma in hard-metal workers: bronchoalveolar lavage, ultrastructural, and analytical findings and results of bronchial provocation tests. Thorax 38, 119–128. 10.1136/thx.38.2.119 - DOI - PMC - PubMed
-
- Drachman JG, Jarvik GP, Mehaffey MG, 2000. Autosomal dominant thrombocytopenia: incomplete megakaryocyte differentiation and linkage to human chromosome 10. Blood 96, 118–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

