A multimodality triage algorithm to improve cytoreductive outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer: A Memorial Sloan Kettering Cancer Center team ovary initiative
- PMID: 32518012
- PMCID: PMC7487016
- DOI: 10.1016/j.ygyno.2020.05.041
A multimodality triage algorithm to improve cytoreductive outcomes in patients undergoing primary debulking surgery for advanced ovarian cancer: A Memorial Sloan Kettering Cancer Center team ovary initiative
Abstract
Objective: To describe outcomes using a multimodal algorithm to triage patients with advanced epithelial ovarian cancer (EOC) to primary debulking surgery (PDS) versus neoadjuvant chemotherapy (NACT).
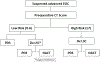
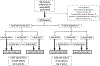
Methods: All patients with EOC treated at our institution from 04/2015-08/2018 were identified. We included patients without contraindication to PDS who underwent prospective calculation of a Resectability (R)-score. A low risk score for suboptimal cytoreduction was defined as ≤6, and a high risk score ≥7. Patients were triaged to laparotomy/PDS, laparoscopic evaluation of resectability (LSC), or NACT depending on R-score.
Results: Among 299 participants, 226 (76%) had a low risk score and 73 (24%) a high risk score. For patients with a low risk score, management included laparotomy/PDS, 181 (80%); LSC, 43 (19%) (with subsequent triage: PDS, 31; NACT, 12); and NACT, 2 (1%). For patients with a high risk score, management included laparotomy/PDS, 9 (12%); LSC, 51 (70%) (with subsequent triage: PDS, 28; NACT, 23); and NACT, 13 (18%). Overall, 83% underwent PDS, with a 75% CGR rate and 94% optimal cytoreduction rate. Use of the algorithm resulted in a 31% LSC rate and a 6% rate of suboptimal PDS.
Conclusions: The multimodal algorithm led to excellent surgical results; 94% of patients achieved an optimal resection, with a very low rate of suboptimal cytoreduction.
Keywords: Cytoreductive outcomes; Neoadjuvant chemotherapy; Ovarian cancer; Primary debulking surgery; Resectability score; Triage algorithm.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Outside the submitted work, Dr. Straubhar has a patent W02019195097A1 perineal heating device issued. Dr. Lakhman is a shareholder of Y-mAbs Therapeutics, Inc. Dr. Abu-Rustum reports grants from Stryker/Novadaq, Olympus, and GRAIL. Dr. Chi reports personal fees from Bovie Medical Co. (now Apyx Medical), Verthermia Inc., C Surgeries, and Biom Up, as well as other from Intuitive Surgical, Inc. and TransEnterix, Inc.
Figures
References
-
- Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. The New England journal of medicine 2010;363:943–53. - PubMed
-
- Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet (London, England) 2015;386:249–57. - PubMed
-
- Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer 2016;64:22–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

