Cell-size regulation in budding yeast does not depend on linear accumulation of Whi5
- PMID: 32518113
- PMCID: PMC7321981
- DOI: 10.1073/pnas.2001255117
Cell-size regulation in budding yeast does not depend on linear accumulation of Whi5
Abstract
Cells must couple cell-cycle progress to their growth rate to restrict the spread of cell sizes present throughout a population. Linear, rather than exponential, accumulation of Whi5, was proposed to provide this coordination by causing a higher Whi5 concentration in cells born at a smaller size. We tested this model using the inducible GAL1 promoter to make the Whi5 concentration independent of cell size. At an expression level that equalizes the mean cell size with that of wild-type cells, the size distributions of cells with galactose-induced Whi5 expression and wild-type cells are indistinguishable. Fluorescence microscopy confirms that the endogenous and GAL1 promoters produce different relationships between Whi5 concentration and cell volume without diminishing size control in the G1 phase. We also expressed Cln3 from the GAL1 promoter, finding that the spread in cell sizes for an asynchronous population is unaffected by this perturbation. Our findings indicate that size control in budding yeast does not fundamentally originate from the linear accumulation of Whi5, contradicting a previous claim and demonstrating the need for further models of cell-cycle regulation to explain how cell size controls passage through Start.
Keywords: Start; Whi5; budding yeast; cell-size control; single-cell time-lapse microscopy.
Conflict of interest statement
The authors declare no competing interest.
Figures

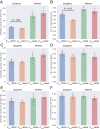
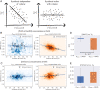
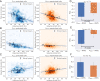
References
-
- Donachie W. D., Relationship between cell size and time of initiation of DNA replication. Nature 219, 1077–1079 (1968). - PubMed
-
- Nurse P., Genetic control of cell size at cell division in yeast. Nature 256, 547–551 (1975). - PubMed
-
- Johnston G. C., Pringle J. R., Hartwell L. H., Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp. Cell Res. 105, 79–98 (1977). - PubMed
-
- Eun Y.-J. et al. ., Archaeal cells share common size control with bacteria despite noisier growth and division. Nat. Microbiol. 3, 148–154 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

