Characteristics of registered clinical trials assessing treatments for COVID-19: a cross-sectional analysis
- PMID: 32518212
- PMCID: PMC7286283
- DOI: 10.1136/bmjopen-2020-039978
Characteristics of registered clinical trials assessing treatments for COVID-19: a cross-sectional analysis
Abstract
Objectives: The coronavirus disease 2019 (COVID-19) pandemic has prompted many initiatives to identify safe and efficacious treatments, yet little is known regarding where early efforts have focused. We aimed to characterise registered clinical trials assessing drugs or plasma treatments for COVID-19.
Design, setting and participants: Cross-sectional analysis of clinical trials for the treatment of COVID-19 that were registered in the USA or in countries contributing to the WHO's International Clinical Trials Registry Platform. Relevant trial entries of drugs or plasma were downloaded on 26 March 2020, deduplicated, verified with reviews of major medical journals and WHO websites and independently analysed by two reviewers.
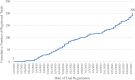
Main outcomes: Trial intervention, sponsorship, critical design elements and specified outcomes RESULTS: Overall, 201 clinical trials were registered for testing the therapeutic benefits of 92 drugs or plasma, including 64 in monotherapy and 28 different combinations. Only eight (8.7%) products or combinations involved new molecular entities. The other test therapies had a wide range of prior medical uses, including as antivirals, antimalarials, immunosuppressants and oncology treatments. In 152 trials (75.7%), patients were randomised to treatment or comparator, including 55 trials with some form of blinding and 97 open-label studies. The 49 (24.4%) of trials without a randomised design included 29 single armed studies and 20 trials with some comparison group. Most trial designs featured multiple endpoints. Clinical endpoints were identified in 134 (66.7%) of trials and included COVID-19 symptoms, death, recovery, required intensive care and hospital discharge. Clinical scales were being used in 33 (16.4%) trials, most often measures of oxygenation and critical illness. Surrogate endpoints or biomarkers were studied in 88 (42.3%) of trials, primarily assays of viral load. Although the trials were initiated in more than 17 countries or regions, 100 (49.8%) were registered in China and 78 (37.8%) in the USA. Registered trials increased rapidly, with the number of registered trials doubling from 1 March to 26 March 2020.
Conclusions: While accelerating morbidity and mortality from the COVID-19 pandemic has been paralleled by early and rapid clinical investigation, many trials lack features to optimise their scientific value. Global coordination and increased funding of high-quality research may help to maximise scientific progress in rapidly discovering safe and effective treatments.
Keywords: epidemiology; infectious diseases; public health.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GCA is past Chair of Food and Drug Administration’s Peripheral and Central Nervous System Advisory Committee; has served as a paid advisor to IQVIA; is a cofounding principal and equity holder in Monument Analytics, a health carehealthcare consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Figures

References
-
- World Health Organization Coronavirus disease (COVID-19) Dashboard. Available: https://covid19.who.int/ [Accessed 4 June 2020].
-
- Coronavirus Resource Center Coronavirus COVID-19 global cases by the center for systems science and engineering. Available: https://coronavirus.jhu.edu/map.html [Accessed 4 June 2020].
-
- Zimmer C. Scientists identify 69 drugs to test against the coronavirus: New York times, 2020. Available: https://www.nytimes.com/2020/03/22/science/coronavirus-drugs-chloroquine... [Accessed 25 Mar 2020].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
