The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation
- PMID: 32518419
- PMCID: PMC7282206
- DOI: 10.1038/s41375-020-0891-0
The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation
Abstract
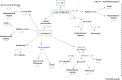
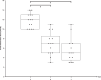
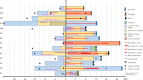
A subgroup of patients with severe COVID-19 suffers from progression to acute respiratory distress syndrome and multiorgan failure. These patients present with progressive hyperinflammation governed by proinflammatory cytokines. An interdisciplinary COVID-19 work flow was established to detect patients with imminent or full blown hyperinflammation. Using a newly developed COVID-19 Inflammation Score (CIS), patients were prospectively stratified for targeted inhibition of cytokine signalling by the Janus Kinase 1/2 inhibitor ruxolitinib (Rux). Patients were treated with efficacy/toxicity guided step up dosing up to 14 days. Retrospective analysis of CIS reduction and clinical outcome was performed. Out of 105 patients treated between March 30th and April 15th, 2020, 14 patients with a CIS ≥ 10 out of 16 points received Rux over a median of 9 days with a median cumulative dose of 135 mg. A total of 12/14 patients achieved significant reduction of CIS by ≥25% on day 7 with sustained clinical improvement in 11/14 patients without short term red flag warnings of Rux-induced toxicity. Rux treatment for COVID-19 in patients with hyperinflammation is shown to be safe with signals of efficacy in this pilot case series for CRS-intervention to prevent or overcome multiorgan failure. A multicenter phase-II clinical trial has been initiated (NCT04338958).
Conflict of interest statement
Nothing to disclose: FL, SB, HCB, MF, MH, BK, IG, AK, SR, Research support by Novartis, Incyte: AH, Adboard Novartis, Incyte: PL; Speakers office: PL.
Figures

Comment in
-
Holding CoVID in check through JAK? The MPN-approved compound ruxolitinib as a potential strategy to treat SARS-CoV-2 induced systemic hyperinflammation.Leukemia. 2020 Jul;34(7):1723-1725. doi: 10.1038/s41375-020-0898-6. Epub 2020 Jun 11. Leukemia. 2020. PMID: 32528040 Free PMC article. No abstract available.
-
Ruxolitinib for the treatment of SARS-CoV-2 induced acute respiratory distress syndrome (ARDS).Leukemia. 2020 Aug;34(8):2276-2278. doi: 10.1038/s41375-020-0907-9. Epub 2020 Jun 17. Leukemia. 2020. PMID: 32555296 Free PMC article. No abstract available.
-
The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19.Leukemia. 2020 Oct;34(10):2815-2816. doi: 10.1038/s41375-020-01038-8. Epub 2020 Sep 2. Leukemia. 2020. PMID: 32879427 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

