Follow-up after surgical treatment for intermittent claudication (FASTIC): a study protocol for a multicentre randomised controlled clinical trial
- PMID: 32518518
- PMCID: PMC7271447
- DOI: 10.1186/s12912-020-00437-7
Follow-up after surgical treatment for intermittent claudication (FASTIC): a study protocol for a multicentre randomised controlled clinical trial
Abstract
Background: Intermittent claudication (IC) is a classic symptom of peripheral arterial disease, and strongly associated with coronary heart disease and cerebrovascular disease. Treatment of IC and secondary prevention of vascular events include best medical treatment (BMT), changes in lifestyle, most importantly smoking cessation and increased physical exercise, and in appropriate cases surgery. A person-centred and health promotion approach might facilitate breaking barriers to lifestyle changes and increasing adherence to secondary prevention therapy. The FASTIC study aims to evaluate a nurse-led, person-centred, health-promoting follow-up programme compared with standard follow-up by a vascular surgeon after surgical treatment for IC.
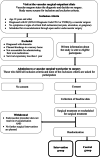
Methods: The FASTIC-study is a multicentre randomised controlled clinical trial. Patients will be recruited from two hospitals in Stockholm, Sweden after surgical treatment of IC through open and/or endovascular revascularisation and will be randomly assigned into two groups. The intervention group is offered a nurse-led, person-centred, health-promoting programme, which includes two telephone calls and three visits to a vascular nurse the first year after surgical treatment. The control group is offered standard care, which consists of a visit to a vascular surgeon 4-8 weeks after surgery and a visit to the outpatient clinic 1 year after surgical treatment. The primary outcome is adherence to BMT 1 year after surgical treatment and will be measured using The Swedish Prescribed Drug Registry. Clinical assessments, biomarkers, and questionnaires will be used to evaluate several secondary outcomes, such as predicted 10-year risk of cardiovascular and cerebrovascular events, health-related quality of life, and patients' perceptions of care quality.
Discussion: The FASTIC study will provide important information about interventions aimed at improving adherence to medication, which is an unexplored field among patients with IC. The study will also contribute to knowledge on how to implement person-centred care in a clinical context.
Trial registration: ClinicalTrials.govNCT03283358, registration date 06/13/2016.
Keywords: Intermittent claudication; Medication adherence; Person-centred care; Randomised controlled trial, atherosclerosis; Secondary prevention; Vascular nursing.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThere are no competing interests to declare for any of the authors.
Figures



References
-
- Kiernan TJ, Hynes BG, Ruggiero NJ, Yan BP, Jaff MR. Comprehensive evaluation and medical management of infrainguinal peripheral artery disease: "when to treat, when not to treat". Tech Vasc Interv Radiol. 2010;13:2–10. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. - PubMed
-
- Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, Powe NR, Siscovick D. Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. The cardiovascular health study group. Arterioscler Thromb Vasc Biol. 1999;19:538–545. - PubMed
-
- Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, Wahlberg E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–1191. - PubMed
-
- Egberg L, Andreassen S, Mattiasson AC. Experiences of living with intermittent claudication. J Vasc Nurs. 2012;30:5–10. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

