Progressive vasoconstriction with sequential thermal stimulation indicates vascular dysautonomia in sickle cell disease
- PMID: 32518948
- PMCID: PMC7472716
- DOI: 10.1182/blood.2020005045
Progressive vasoconstriction with sequential thermal stimulation indicates vascular dysautonomia in sickle cell disease
Abstract
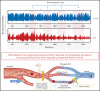
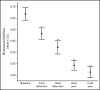
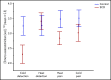
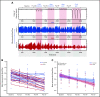
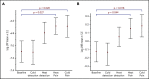
Persons with sickle cell disease (SCD) exhibit subjective hypersensitivity to cold and heat perception in experimental settings, and triggers such as cold exposure are known to precipitate vaso-occlusive crises by still unclear mechanisms. Decreased microvascular blood flow (MBF) increases the likelihood of vaso-occlusion by increasing entrapment of sickled red blood cells in the microvasculature. Because those with SCD have dysautonomia, we anticipated that thermal exposure would induce autonomic hypersensitivity of their microvasculature with an increased propensity toward vasoconstriction. We exposed 17 patients with SCD and 16 control participants to a sequence of predetermined threshold temperatures for cold and heat detection and cold and heat pain via a thermode placed on the right hand. MBF was measured on the contralateral hand by photoplethysmography, and cardiac autonomic balance was assessed by determining heart rate variability. Thermal stimuli at both detection and pain thresholds caused a significant decrease in MBF in the contralateral hand within seconds of stimulus application, with patients with SCD showing significantly stronger vasoconstriction (P = .019). Furthermore, patients with SCD showed a greater progressive decrease in blood flow than did the controls, with poor recovery between episodes of thermal stimulation (P = .042). They had faster vasoconstriction than the controls (P = .033), especially with cold detection stimulus. Individuals with higher anxiety also experienced more rapid vasoconstriction (P = .007). Augmented vasoconstriction responses and progressive decreases in perfusion with repeated thermal stimulation in SCD are indicative of autonomic hypersensitivity in the microvasculature. These effects are likely to increase red cell entrapment in response to clinical triggers such as cold or stress, which have been associated with vaso-occlusive crises in SCD.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


Comment in
-
Vasomotor hyperresponsiveness in SCD.Blood. 2020 Sep 3;136(10):1120-1121. doi: 10.1182/blood.2020007070. Blood. 2020. PMID: 32882017 No abstract available.
References
-
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018-2031. - PubMed
-
- Kassim AA, DeBaun MR. Sickle cell disease, vasculopathy, and therapeutics. Annu Rev Med. 2013;64(1):451-466. - PubMed
-
- Platt OS, Thorington BD, Brambilla DJ, et al. . Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11-16. - PubMed
-
- Serjeant GR. Natural history and determinants of clinical severity of sickle cell disease. Curr Opin Hematol. 1995;2(2):103-108. - PubMed
-
- Steinberg MH, H SM. Predicting clinical severity in sickle cell anaemia. Br J Haematol. 2005;129(4):465-481. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

