Polidocanol versus phenol in oil injection sclerotherapy in treatment of internal hemorrhoids: A randomized controlled trial
- PMID: 32519957
- PMCID: PMC7289163
- DOI: 10.5152/tjg.2020.19276
Polidocanol versus phenol in oil injection sclerotherapy in treatment of internal hemorrhoids: A randomized controlled trial
Abstract
Background/aims: Management of Haemorrhoids is suboptimal and is largely based on traditional practices in the Indian population. Though injection sclerotherapy is a well-accepted treatment modality in early grade haemorrhoids, there is no consensus on the effectiveness of the drugs used for sclerotherapy. The study was done to compare the safety and efficacy of a standard sclerosant (polidocanol) and the conventionally used phenol in oil in bleeding grade-1 and 2 internal haemorrhoids.
Materials and methods: All patients with grade-1 and 2 hemorrhoids, were selected and randomised into two groups, 3% polidocanol and 5% phenol group. All patients were followed-up for three months and observed for "free of bleeding" or "persistent bleeding." Pain, pruritus and patient satisfaction following the procedure was also assessed.
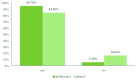
Results: A total of 150 patients were enrolled, 75 in each group. At the end of the first sclerotherapy session with polidocanol, 60.6% of patients versus 38.1% in phenol group had stopped per rectal bleeding (p=0.009). After the second sclerotherapy session, 94.7% of patients in the polidocanol group and 84% of patients in the phenol group were treated successfully. Polidocanol group required significantly fewer treatment sessions than the phenol group (1.39±0.49 vs. 1.62±0.49; p=0.035), and the total volume of injected sclerosant was also less (3.30±0.96 mL vs. 4.86±1.46 mL; p=0.001). The patient satisfaction was 87% in polidocanol group versus 73% in phenol group (p=0.040).
Conclusion: 3% polidocanol is safe and more effective than 5% phenol in oil when used as injection sclerotherapy in the treatment of first and second-degree internal hemorrhoids.
Conflict of interest statement
Figures
References
-
- Ali ZH, El-Sayed NO, Taha NM. Effect of conservative measures in improving hemorrhoid stages and relieving symptoms among patients with haemorrhoids. J Am Sci. 2011;7:53–65.
-
- Khubchandani I, Paonessa N, Khwaja A. Surgical Treatment of Hemorrhoids. 2nd ed. London: Springer; 2009. p. 185. - DOI
-
- Moser KH, Mosch C, Walgenbach M, et al. Efficacy and safety of sclerotherapy with polidocanol foam in comparison with fluid sclerosant in the treatment of first-grade haemorrhoidal disease: a randomised, controlled, single-blind, multicentre trial. Int J Colorectal Dis. 2013;28:1439–47. doi: 10.1007/s00384-013-1729-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical