Association of Naturalistic Administration of Cannabis Flower and Concentrates With Intoxication and Impairment
- PMID: 32520316
- PMCID: PMC7287943
- DOI: 10.1001/jamapsychiatry.2020.0927
Association of Naturalistic Administration of Cannabis Flower and Concentrates With Intoxication and Impairment
Abstract
Importance: The rapidly growing legal cannabis market includes new and highly potent products, the effects of which, to our knowledge, have not previously been examined in biobehavioral research studies because of federal restrictions on cannabis research.
Objective: To use federally compatible, observational methods to study high-∆9-tetrahydrocannabinol (THC) legal market forms of cannabis.
Design, setting, and participants: In this cohort study with a between-groups design that was conducted in a community and university setting, cannabis flower users and concentrate users were randomly assigned to higher- vs lower-THC products within user groups. Participants completed a baseline and an experimental mobile laboratory assessment that included 3 points: before, immediately after, and 1 hour after ad libitum legal market flower and concentrate use. Of the 133 individuals enrolled and assessed, 55 regular flower cannabis users (41.4%) and 66 regular concentrate cannabis users (49.6%) complied with the study's cannabis use instructions and had complete data across primary outcomes.
Exposures: Flower users were randomly assigned to use either 16% or 24% THC flower and concentrate users were randomly assigned to use either 70% or 90% THC concentrate that they purchased from a dispensary.
Main outcomes and measures: Primary outcome measures included plasma cannabinoids, subjective drug intoxication, and neurobehavioral tasks testing attention, memory, inhibitory control, and balance.
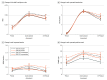
Results: A total of 121 participants completed the study for analysis: 55 flower users (mean [SD] age, 28.8 [8.1] years; 25 women [46%]) and 66 concentrate users (mean [SD] age, 28.3 [10.4] years; 30 women [45%]). Concentrate users compared with flower users exhibited higher plasma THC levels and 11-hydroxyΔ9-THC (THC's active metabolite) across all points. After ad libitum cannabis administration, mean plasma THC levels were 0.32 (SE = 0.43) μg/mL in concentrate users (to convert to millimoles per liter, multiply by 3.18) and 0.14 (SE = 0.16) μg/mL in flower users. Most neurobehavioral measures were not altered by short-term cannabis consumption. However, delayed verbal memory (F1,203 = 32.31; P < .001) and balance function (F1,203 = 18.88; P < .001) were impaired after use. Differing outcomes for the type of product (flower vs concentrate) or potency within products were not observed.
Conclusions and relevance: This study provides information about the association of pharmacological and neurobehavioral outcomes with legal market cannabis. Short-term use of concentrates was associated with higher levels of THC exposure. Across forms of cannabis and potencies, users' domains of verbal memory and proprioception-focused postural stability were primarily associated with THC administration.
Conflict of interest statement
Figures


References
-
- Orens A, Light M, Lewandowski B, Rowberry J, Saloga C 2017 market update. Accessed August 20, 2018. https://www.colorado.gov/pacific/sites/default/files/MED%20Demand%20and%...

