First-in-human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple oral doses of SAR247799, a selective G-protein-biased sphingosine-1 phosphate receptor-1 agonist for endothelial protection
- PMID: 32520410
- PMCID: PMC9328431
- DOI: 10.1111/bcp.14422
First-in-human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple oral doses of SAR247799, a selective G-protein-biased sphingosine-1 phosphate receptor-1 agonist for endothelial protection
Abstract
Aims: SAR247799 is a selective G-protein-biased sphingosine-1 phosphate receptor-1 (S1P1 ) agonist with potential to restore endothelial function in vascular pathologies. SAR247799, a first-in-class molecule differentiated from previous S1P1 -desensitizing molecules developed for multiple sclerosis, can activate S1P1 without desensitization and consequent lymphopenia. The aim was to characterize SAR247799 for its safety, tolerability, pharmacokinetics and pharmacodynamics (activation and desensitization).
Methods: SAR247799 was administered orally to healthy subjects in a double-blind, randomized, placebo-controlled study with single (2.5-37.5 mg) or 2-week once-daily (0.5-15 mg) doses. An open-label single dose pilot food-interaction arm with 10 mg SAR247799 in cross-over design was also performed.
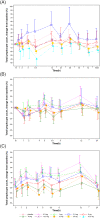
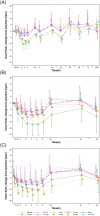
Results: SAR247799 was well tolerated and, at the higher end of the dose ranges, caused the expected dose-dependent pharmacodynamics associated with S1P1 activation (heart rate reduction) and S1P1 desensitization (lymphocyte count reduction). SAR247799 demonstrated dose-proportional increases in exposure and was eliminated with an apparent terminal half-life of 31.2-33.1 hours. Food had a small effect on the pharmacokinetics of SAR247799. SAR247799 had a low volume of distribution (7-23 L), indicating a potential to achieve dose separation for endothelial vs cardiac S1P1 activation pharmacology. A supratherapeutic dose (10 mg) of SAR247799 produced sustained heart rate reduction over 14 days, demonstrating cardiac S1P1 activation without tachyphylaxis. Sub-lymphocyte-reducing doses (≤5 mg) of SAR247799, which, based on preclinical data, are projected to activate S1P1 and exhibit endothelial-protective properties, had minimal-to-no heart rate reduction and displayed no marked safety findings.
Conclusion: SAR247799 is suitable for exploring the biological role of endothelial S1P1 activation without causing receptor desensitization.
Keywords: SAR247799; endothelium; pharmacodynamics; pharmacokinetics; sphingosine 1-phosphate.
© 2020 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Authors were employees of Sanofi or Paraxel at the time of conduct of the studies. Authors affiliated with Sanofi may have equity interest in Sanofi. A.A.P. is inventor of US patent number 9,782,411.
Figures


References
-
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Pharmacol Ther. 2007;115:84‐105. - PubMed
-
- Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355‐360. - PubMed
-
- Gilenya (fingolimod) package insert . Basel, Switzerland. Novartis pharmaceuticals 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

