Impact of Routine Point-of-Care Versus Laboratory Testing for Early Infant Diagnosis of HIV: Results From a Multicountry Stepped-Wedge Cluster-Randomized Controlled Trial
- PMID: 32520909
- PMCID: PMC7302335
- DOI: 10.1097/QAI.0000000000002383
Impact of Routine Point-of-Care Versus Laboratory Testing for Early Infant Diagnosis of HIV: Results From a Multicountry Stepped-Wedge Cluster-Randomized Controlled Trial
Abstract
Background: Although the World Health Organization recommends HIV-exposed infants receive a 6-week diagnostic test, few receive results by 12 weeks. Point-of-care (POC) early infant diagnosis (EID) may improve timely diagnosis and treatment. This study assesses the impact of routine POC versus laboratory-based EID on return of results by 12 weeks of age.
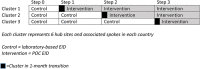
Methods: This was a cluster-randomized stepped-wedge trial in Kenya and Zimbabwe. In each country, 18 health facilities were randomly selected for inclusion and randomized to timing of POC implementation.
Findings: Nine thousand five hundred thirty-nine infants received tests: 5115 laboratory-based and 4424 POC. In Kenya and Zimbabwe, respectively, caregivers were 1.29 times [95% confidence interval (CI): 1.27 to 1.30, P < 0.001] and 4.56 times (95% CI: 4.50 to 4.60, P < 0.001) more likely to receive EID results by 12 weeks of age with POC versus laboratory-based EID. POC significantly reduced the time between sample collection and return of results to caregiver by an average of 23.03 days (95% CI: 4.85 to 21.21, P < 0.001) in Kenya and 62.37 days (95% CI: 58.94 to 65.80, P < 0.001) in Zimbabwe. For HIV-infected infants, POC significantly increased the percentage initiated on treatment, from 43.2% to 79.6% in Zimbabwe, and resulted in a nonsignificant increase in Kenya from 91.7% to 100%. The introduction of POC EID also significantly reduced the time to antiretroviral therapy initiation by an average of 17.01 days (95% CI: 9.38 to 24.64, P < 0.001) in Kenya and 56.00 days (95% CI: 25.13 to 153.76, P < 0.001) in Zimbabwe.
Conclusions: POC confers significant advantage on the proportion of caregivers receiving timely EID results, and improves time to results receipt and treatment initiation for infected infants. Where laboratory-based EID systems are unable to deliver results to caregivers rapidly, POC should be implemented as part of an integrated testing system.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- UNAIDS. Global AIDS Update. 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-global-AIDS-.... Accessed January 1, 2020.
-
- Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23:101–106. - PubMed
-
- Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. - PubMed
-
- WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV infection. Recommendations for a Public Health Approach. 2016. Available at: https://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed January 1, 2020. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical