Strengthening Existing Laboratory-Based Systems vs. Investing in Point-of-Care Assays for Early Infant Diagnosis of HIV: A Model-Based Cost-Effectiveness Analysis
- PMID: 32520910
- PMCID: PMC7302325
- DOI: 10.1097/QAI.0000000000002384
Strengthening Existing Laboratory-Based Systems vs. Investing in Point-of-Care Assays for Early Infant Diagnosis of HIV: A Model-Based Cost-Effectiveness Analysis
Abstract
Background: To improve early infant HIV diagnosis (EID) programs, options include replacing laboratory-based tests with point-of-care (POC) assays or investing in strengthened systems for sample transport and result return.
Setting: We used the CEPAC-Pediatric model to examine clinical benefits and costs of 3 EID strategies in Zimbabwe for infants 6 weeks of age.
Methods: We examined (1) laboratory-based EID (LAB), (2) strengthened laboratory-based EID (S-LAB), and (3) POC EID (POC). LAB/S-LAB and POC assays differed in sensitivity (LAB/S-LAB 100%, POC 96.9%) and specificity (LAB/S-LAB 99.6%, POC 99.9%). LAB/S-LAB/POC algorithms also differed in: probability of result return (79%/91%/98%), time until result return (61/53/1 days), probability of initiating antiretroviral therapy (ART) after positive result (52%/71%/86%), and total cost/test ($18.10/$30.47/$30.71). We projected life expectancy (LE) and average lifetime per-person cost for all HIV-exposed infants. We calculated incremental cost-effectiveness ratios (ICERs) from discounted (3%/year) LE and costs in $/year-of-life saved (YLS), defining cost effective as an ICER <$580/YLS (reflecting programs providing 2 vs. 1 ART regimens). In sensitivity analyses, we varied differences between S-LAB and POC in result return probability, result return time, ART initiation probability, and cost.
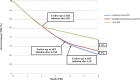
Results: For infants who acquired HIV, LAB/S-LAB/POC led to projected one-year survival of 67.3%/69.9%/75.6% and undiscounted LE of 21.74/22.71/24.49 years. For all HIV-exposed infants, undiscounted LE was 63.35/63.38/63.43 years, at discounted lifetime costs of $200/220/240 per infant. In cost-effectiveness analysis, S-LAB was an inefficient use of resources; the ICER of POC vs. LAB was $830/YLS.
Conclusions: Current EID programs will attain greater benefit from investing in POC EID rather than strengthening laboratory-based systems.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

Similar articles
-
Clinical effect and cost-effectiveness of incorporation of point-of-care assays into early infant HIV diagnosis programmes in Zimbabwe: a modelling study.Lancet HIV. 2019 Mar;6(3):e182-e190. doi: 10.1016/S2352-3018(18)30328-X. Epub 2019 Feb 5. Lancet HIV. 2019. PMID: 30737187 Free PMC article.
-
Optimizing infant HIV diagnosis with additional screening at immunization clinics in three sub-Saharan African settings: a cost-effectiveness analysis.J Int AIDS Soc. 2021 Jan;24(1):e25651. doi: 10.1002/jia2.25651. J Int AIDS Soc. 2021. PMID: 33474817 Free PMC article.
-
The clinical and economic impact of point-of-care CD4 testing in mozambique and other resource-limited settings: a cost-effectiveness analysis.PLoS Med. 2014 Sep 16;11(9):e1001725. doi: 10.1371/journal.pmed.1001725. eCollection 2014 Sep. PLoS Med. 2014. PMID: 25225800 Free PMC article.
-
Cost-effectiveness of point-of-care versus centralised, laboratory-based nucleic acid testing for diagnosis of HIV in infants: a systematic review of modelling studies.Lancet HIV. 2023 May;10(5):e320-e331. doi: 10.1016/S2352-3018(23)00029-2. Lancet HIV. 2023. PMID: 37149292 Free PMC article.
-
Systematic review of the performance and clinical utility of point of care HIV-1 RNA testing for diagnosis and care.PLoS One. 2019 Jun 27;14(6):e0218369. doi: 10.1371/journal.pone.0218369. eCollection 2019. PLoS One. 2019. PMID: 31246963 Free PMC article.
Cited by
-
Programmatic evaluation of feasibility and efficiency of at birth and 6-week, point of care HIV testing in Kenyan infant.PLoS One. 2020 Oct 9;15(10):e0240621. doi: 10.1371/journal.pone.0240621. eCollection 2020. PLoS One. 2020. PMID: 33035274 Free PMC article.
-
Costs and cost-effectiveness of HIV early infant diagnosis in low- and middle-income countries: a scoping review.Infect Dis Poverty. 2022 Jul 15;11(1):82. doi: 10.1186/s40249-022-01006-7. Infect Dis Poverty. 2022. PMID: 35841117 Free PMC article.
-
Incorporating the HIV Infant Tracking System into standard-of-care early infant diagnosis of HIV services in Kenya: a cost-effectiveness analysis of the HITSystem randomised trial.Lancet Glob Health. 2023 Aug;11(8):e1217-e1224. doi: 10.1016/S2214-109X(23)00216-4. Lancet Glob Health. 2023. PMID: 37474229 Free PMC article. Clinical Trial.
-
Uganda's "EID Systems Strengthening" model produces significant gains in testing, linkage, and retention of HIV-exposed and infected infants: An impact evaluation.PLoS One. 2021 Feb 4;16(2):e0246546. doi: 10.1371/journal.pone.0246546. eCollection 2021. PLoS One. 2021. PMID: 33539425 Free PMC article.
-
Using queueing models as a decision support tool in allocating point-of-care HIV viral load testing machines in Kisumu County, Kenya.Health Policy Plan. 2024 Jan 9;39(1):44-55. doi: 10.1093/heapol/czad111. Health Policy Plan. 2024. PMID: 37949109 Free PMC article.
References
-
- Unicef. Progress in Reducing New HIV Infections Among Children Has Been Made, but Not Fast Enough. 2019. Available at: https://data.unicef.org/topic/hivaids/emtct/. Accessed November 12, 2019.
-
- Only half of HIV-exposed babies are tested for HIV. UNAIDS. 2019. Available at: https://www.unaids.org/en/resources/presscentre/featurestories/2019/marc.... Accessed October 10, 2019.
-
- World Health Organization. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines. 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/277395/WHO-CDS-HIV-18.5.... Accessed October 12, 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

