Propensity score analysis of the prognostic value of genomic assays for breast cancer in diverse populations using the National Cancer Data Base
- PMID: 32521056
- PMCID: PMC7423613
- DOI: 10.1002/cncr.32956
Propensity score analysis of the prognostic value of genomic assays for breast cancer in diverse populations using the National Cancer Data Base
Abstract
Background: Genomic assays such as Oncotype Dx (ODX) and MammaPrint are used for risk-adapted treatment decisions among patients with early breast cancer. However, to the authors' knowledge, concordance between genomic assays is modest. Using real-world data, the authors performed a comparative analysis of ODX and MammaPrint.
Methods: A cohort of women diagnosed with early-stage, hormone receptor-positive breast cancer who received ODX or MammaPrint was established using the National Cancer Data Base (NCDB) for 2010 through 2016. Using the propensity score matching method, 2 groups of patients with similar clinical and demographic characteristics were defined: one group received ODX and the other received MammaPrint. The authors examined the association between use of the ODX or MammaPrint assays and overall survival using Cox models.
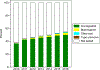
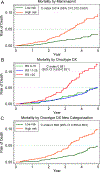
Results: Of the 451,693 eligible patients, approximately 45.3% received ODX and 1.8% received MammaPrint testing. The use of ODX increased from 36.1% in 2010 to 49.9% in 2016, whereas use of MammaPrint increased from 0.5% in 2010 to 3.3% in 2016. The authors matched 5042 patients who received ODX with 5042 patients who received MammaPrint. The 5-year risks of death for the MammaPrint low-risk group and the ODX low-risk group were 3.4% and 4.7%, respectively. The prognostic value of MammaPrint was similar to that of ODX; the C-index was 0.614 (95% confidence interval, 0.572-0.657) for MammaPrint and 0.581 (95% confidence interval, 0.530-0.631) for ODX. There was a difference in the performance of the ODX assay observed across racial and/or ethnic groups (P < .001), with a slightly better performance noted among white compared with African American and Hispanic individuals.
Conclusions: Both the ODX and MammaPrint tests are good at identifying low-risk individuals who could be spared chemotherapy. The suboptimal performance of ODX in ethnic minority individuals deserves further investigation.
Keywords: biomarker; breast cancer; endocrine receptor; genomic assay; racial disparity.
© 2020 American Cancer Society.
Conflict of interest statement
The authors had no conflict of interest.
Figures
References
-
- Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? Journal of Clinical Oncology. 2005;23: 7350–7360. - PubMed
-
- Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. The Oncologist. 2007;12: 631–635. - PubMed
-
- Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. New England Journal of Medicine. 2004;351: 2817–2826. - PubMed
-
- Ramsey SD, Barlow WE, Gonzalez-Angulo AM, et al. Integrating comparative effectiveness design elements and endpoints into a phase III, randomized clinical trial (SWOG S1007) evaluating oncotypeDX-guided management for women with breast cancer involving lymph nodes. Contemporary clinical trials. 2013;34: 1–9. - PMC - PubMed
-
- Van’t Veer LJ, Dai H, Van De Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415: 530. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

